Supramolecular Catalyzed Cascade Reduction of Azaarenes Interrogated via Data Science
- PMID: 39432827
- PMCID: PMC11528432
- DOI: 10.1021/jacs.4c11482
Supramolecular Catalyzed Cascade Reduction of Azaarenes Interrogated via Data Science
Abstract
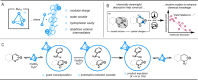
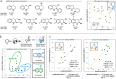
Catalysis of multicomponent transformations requires controlled assembly of reactants within the active site. Supramolecular scaffolds possess synthetic microenvironments that enable precise modulation over noncovalent interactions (NCIs) engaged by reactive, encapsulated species. While molecular properties that describe the behavior of single guests in host cavities have been studied extensively, multicomponent transformations remain challenging to design and deploy. Here, simple univariate regression and threshold analyses are employed to model reactivity in a cascade reduction of azaarenes catalyzed by water-soluble metal organic cages. Yield and stereoselectivity models help deduce unknown mechanisms of reactivity by the multicomponent, host-guest complexes. Furthermore, a comprehensive model is established for NCIs driving stereoselectivity in the reported host-guest adducts.
Conflict of interest statement
The authors declare no competing financial interest.
Figures