DKN-01 in Combination With Tislelizumab and Chemotherapy as First-Line Therapy in Advanced Gastric or Gastroesophageal Junction Adenocarcinoma: DisTinGuish
- PMID: 39432867
- PMCID: PMC11771358
- DOI: 10.1200/JCO.24.00410
DKN-01 in Combination With Tislelizumab and Chemotherapy as First-Line Therapy in Advanced Gastric or Gastroesophageal Junction Adenocarcinoma: DisTinGuish
Abstract
Purpose: The outcomes of anti-PD-1 agents plus fluoropyrimidine/platinum in frontline advanced gastroesophageal adenocarcinomas (aGEAs) remain poor. We investigated the safety, tolerability, and activity of fluoropyrimidine/oxaliplatin and tislelizumab with the DKK1-neutralizing antibody DKN-01 in aGEAs in a phase IIa open-label study.
Patients and methods: Patients had untreated human epidermal growth factor receptor 2-negative aGEAs, RECIST v1.1 measurable disease, Eastern Cooperative Oncology Group (ECOG) performance status 0-1, and adequate organ function. Patients received intravenous DKN-01 300 mg once every 2 weeks, tislelizumab 200 mg once every 3 weeks, oxaliplatin 130 mg/m2 once every 3 weeks, and capecitabine 1,000 mg/m2 twice daily on days 1-15 of each 21-day cycle. The primary end point was safety and tolerability. Key secondary end points included objective response rate (ORR) by RECISTv1.1, progression-free survival (PFS), and overall survival (OS).
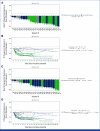
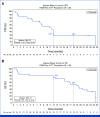
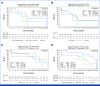
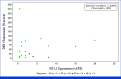
Results: Between September 18, 2020, and April 8, 2021, 25 patients were enrolled. All patients who received at least one dose of DKN-01 were included in the safety analysis. Most patients had gastroesophageal junction tumors, median age was 61 years, 76% were male, and 55% were ECOG of 0. All patients reported at least one treatment-emergent adverse event. The ORR was 73% (95% CI, 49.8 to 89.3), with a disease control rate of 95%. The ORR was 90% (95% CI, 55.5 to 99.7) in the DKK1-high tumor patients and 67% (95% CI, 29.9 to 92.5) in the DKK1-low tumor patients. The median PFS was 11.3 months (95% CI, 5.8 to 12.0) and the 12-month PFS rate was 33%. The median OS was 19.5 months (95% CI, 15.2 to 24.4) with a 12-month OS rate of 76% and an 18-month OS rate of 55%.
Conclusion: DKN-01 can be safely combined with frontline fluoropyrimidine/oxaliplatin and tislelizumab and demonstrates encouraging activity independent of PD-L1 expression levels. A randomized phase II trial is ongoing (ClinicalTrials.gov identifier: NCT04363801).
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures

References
-
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. - PubMed
-
- Rha SY, Oh D-Y, Yañez P, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24:1181–1195. - PubMed
-
- Sun J-M, Shen L, Shah MA, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): A randomised, placebo-controlled, phase 3 study. Lancet. 2021;398:759–771. - PubMed
-
- Kang Y-K, Chen L-T, Ryu M-H, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): A randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23:234–247. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

