Global, regional, and national burden of pulmonary arterial hypertension, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021
- PMID: 39433052
- PMCID: PMC11698691
- DOI: 10.1016/S2213-2600(24)00295-9
Global, regional, and national burden of pulmonary arterial hypertension, 1990-2021: a systematic analysis for the Global Burden of Disease Study 2021
Abstract
Background: Pulmonary arterial hypertension (PAH) is a vascular disease characterised by restricted flow and high pressure through the pulmonary arteries, leading to progressive right heart failure and death. This study reports the global burden of PAH, leveraging all available data and using methodology of the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) to understand the epidemiology of this under-researched and morbid disease.
Methods: Prior to the current effort, the burden of PAH was included in GBD as a non-specific contributor to "other cardiovascular and circulatory disease" burden. In this study, PAH was distinguished as its own cause of death and disability in GBD, producing comparable and consistent estimates of PAH burden. We used epidemiological and vital registry data to estimate the non-fatal and fatal burden of PAH in 204 countries and territories from 1990 to 2021 using standard GBD modelling approaches. We specifically focused on PAH (group 1 pulmonary hypertension), and did not include pulmonary hypertension groups 2-5.
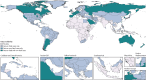
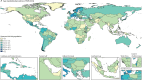
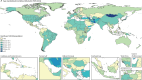
Findings: In 2021, there were an estimated 192 000 (95% uncertainty interval [UI] 155 000-236 000) prevalent cases of PAH globally. Of these, 119 000 (95 900-146 000) were in females (62%) and 73 100 (58 900-89 600) in males (38%). The age-standardised prevalence was 2·28 cases per 100 000 population (95% UI 1·85-2·80). Prevalence increased with age such that the highest prevalence was among individuals aged 75-79 years. In 2021, there were 22 000 deaths (18 200-25 400) attributed to PAH globally, with an age-standardised mortality rate of 0·27 deaths from PAH per 100 000 population (0·23-0·32). The burden of disease appears to be improving over time (38·2% improvement in age-standardised years of life lost [YLLs] in 2021 relative to 1990). YLLs attributed to PAH were similar to estimates for conditions such as chronic myeloid leukaemia, multiple sclerosis, and Crohn's disease.
Interpretation: PAH is a rare but fatal disease that accounts for a considerable health-associated burden worldwide. PAH is disproportionally diagnosed among females and older adults.
Funding: Cardiovascular Medical Research and Education Fund and the Bill & Melinda Gates Foundation.
Copyright © 2025 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests O C Baltatu reports support for the present manuscript from the National Council for Scientific and Technological Development (CNPq, 304224/2022-7) and the Anima Institute through an AI research professor fellowship; and a leadership or fiduciary role with the Health and Biotechnology Advisory Board at Technology Park São José dos Campos – Center for Innovation in Health Technologies (CITS) outside the submitted work. S Bhaskar reports grants or contracts from the Japan Society for the Promotion of Science (JSPS), Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT) through a Grant-in-Aid for Scientific Research (KAKENHI), and from JSPS and the Australian Academy of Science through a JSPS International Fellowship; and leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid, with Rotary District 9675, the Global Health & Migration Hub Community, Global Health Hub Germany, Berlin, with PLOS One, BMC Neurology, Frontiers in Neurology, Frontiers in Stroke, Frontiers in Public Health, and BMC Medical Research Methodology as an Editorial Board Member, and as a member of the College of Reviewers, Canadian Institutes of Health Research (CIHR), Government of Canada; all outside the submitted work. P A Corris reports participation on a data safety monitoring board or advisory board with Aerovate and Merck; and leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid, as Chairman of the Pulmonary Vascular Research Institute; all outside the submitted work. H M DuBrock reports a research grant from Bayer Pharmaceuticals; consulting fees from Merck; and participation on an advisory board with Merck and Janssen Pharmaceuticals; all outside the submitted work. M Hultström reports support for the present manuscript from the Swedish Heart-Lung Foundation and Knut och Alice Wallenberg Foundation through payments to their institution; grants or contracts from the Swedish Society of Medicine through their institution; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from the Swedish Society for Anaesthesiology and Intensive Care Medicine; support for attending meetings or travel from the American Physiological Society; and leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid, with the American Physiological Society as a Steering Committee Member of the section for Water and Electrolyte Homeostasis; all outside the submitted work. N E Ismail reports a leadership or fiduciary role in other board, society, committee or advocacy group, unpaid, with the Malaysian Academy of Pharmacy, Malaysia, and the Malaysian Pharmacists Society Education Chapter outside the submitted work. J J Jozwiak reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Novartis, Adamed, and Amgen, outside the submitted work. K Krishan reports non-financial support from the UGC Centre of Advanced Study, CAS II, awarded to the Department of Anthropology, Panjab University, Chandigarh, India, outside the submitted work. P J Leary reports grants or contracts from the National Heart, Lung, and Blood Institute through payments to them and their institution (R33/R61: Famotidine RCT; R01: Multi-omics; R33/R61: Valsartan; R01: Methamphetamine PAH), from Bayer through payments to their institution (Mentor on a mentored award about FDG-uptake in PAH; no honoraria or salary support), from the Cystic Fibrosis Foundation Therapeutic Development Network through payments to them and their institution, and from Janssen Pharmaceuticals through payments to a third party for data acquisition and analysis; consulting fees from Sumitomo Pharma as personal payments; participation on a data safety monitoring board with the National Heart, Lung, and Blood Institute; leadership or fiduciary role, unpaid, with the Pulmonary Hypertension Association Scientific Leadership Council and the Team Phenomenal Hope Steering Committee; and receipt of medical writing support and collation of co-author feedback on a big-data locations of care project (under review) from Janssen Pharmaceuticals; all outside the submitted work. M-C Li reports grants or contacts from the National Science and Technology Council, Taiwan (NSTC 113-2314-B-003-002); and leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid, with the Journal of the American Heart Association; all outside the submitted work. J A Singh reports consulting fees from ROMTech, Atheneum, ClearView Healthcare Partners, American College of Rheumatology, Yale, Hulio, Horizon Pharmaceuticals, DINORA, Frictionless Solutions, Schipher, Crealta/Horizon, Medisys, Fidia, PK Med, Two Labs Inc, Adept Field Solutions, Clinical Care Options, Putnam Associates, Focus Forward, Navigant Consulting, Spherix, MedIQ, Jupiter Life Science, UBM LLC, Trio Health, Medscape, WebMD, and Practice Point Communications, and the National Institutes of Health; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events on the speakers bureau of Simply Speaking; support for attending meetings or travel from OMERACT as a steering committee member; participation on a data safety monitoring board or advisory board with the FDA Arthritis Advisory Committee; leadership or fiduciary role in other board, society, committee, or advocacy group, paid as a past steering committee member of the OMERACT, an international organisation that develops measures for clinical trials and receives arm's length funding from 12 pharmaceutical companies, unpaid as Chair of the Veterans Affairs Rheumatology Field Advisory Committee, and unpaid as the Editor and Director of the UAB Cochrane Musculoskeletal Group Satellite Center on Network Meta-analysis; stock or stock options in Atai life sciences, Kintara Therapeutics, Intelligent Biosolutions, Acumen Pharmaceutical, TPT Global Tech, Vaxart Pharmaceuticals, Atyu Biopharma, Adaptimmune Therapeutics, GeoVax Labs, Pieris Pharmaceuticals, Enzolytics Inc, Seres Therapeutics, Tonix Pharmaceuticals Holding Corp, Aebona Pharmaceuticals, and Charlotte's Web Holdings, Inc; and previous stock options in Amarin, Viking, and Moderna Pharmaceuticals; all outside the submitted work. C E Ventetuolo reports grants or contracts from the National Institutes of Health and American Heart Association through payments to their institution; consulting fees from Merck, Janssen, United Therapeutics, and Regeneron; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Dynamed; and support for attending meetings or travel from the American Thoracic Society; all outside the submitted work. M Zielińska reports other financial or non-financial interests in AstraZeneca as an employee, outside the submitted work. All other authors declare no competing interests.
Figures


References
-
- Dresdale DT, Schultz M, Michtom RJ. Primary pulmonary hypertension. I. Clinical and hemodynamic study. Am J Med. 1951;11:686–705. - PubMed
-
- Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2023;61 - PubMed
-
- McLaughlin VV, Shah SJ, Souza R, Humbert M. Management of pulmonary arterial hypertension. J Am Coll Cardiol. 2015;65:1976–1997. - PubMed
-
- Gu S, Hu H, Dong H. Systematic review of the economic burden of pulmonary arterial hypertension. PharmacoEconomics. 2016;34:533–550. - PubMed
-
- Noel ZR, Kido K, Macaulay TE. Selexipag for the treatment of pulmonary arterial hypertension. Am J Health Syst Pharm. 2017;74:1135–1141. - PubMed

