Results from Arm A of Phase 1/2 DREAMM-6 trial: belantamab mafodotin with lenalidomide plus dexamethasone in patients with relapsed/refractory multiple myeloma
- PMID: 39433730
- PMCID: PMC11494064
- DOI: 10.1038/s41408-024-01155-y
Results from Arm A of Phase 1/2 DREAMM-6 trial: belantamab mafodotin with lenalidomide plus dexamethasone in patients with relapsed/refractory multiple myeloma
Abstract
Conflict of interest statement
RP is supported by the Biomedical Research Center of the National Institute for Health Research and University College London Hospitals and received speaker honorarium from AbbVie, GSK, Janssen Biotech, Inc., BMS and Pfizer, has participated in advisory boards for GSK and Janssen Biotech, Inc., and has received research funding from GSK and Pfizer. BA has sat on scientific advisory boards for Janssen-Cilag Pty Ltd. MG discloses no conflicts of interest. CL has participated in advisory boards for Janssen-Cilag, BMS, Antengene, Takeda, and Amgen. PC discloses no conflicts of interest. NP, RSK, RR, MS, AC, FC, SR-G, and JO are employees of GSK and hold stocks/shares in GSK. HQ has received research funding from GSK, BMS, Karyopharm, and Abbvie, has received speaker honorarium for Beigene, GSK, and Abbvie and has participated on advisory boards for BMS, Abbvie, GSK, Janssen Cilag, Pfizer, Antengene, Sanofi and Regeneron.
Figures
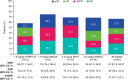
References
-
- De Oca RM, Gupta I, Shelton C. Abstract 6711: Combinations of belantamab mafodotin with lenalidomide, pomalidomide, bortezomib and/or dexamethasone synergize in vitro and potentiate in vivo anti-tumor activity in multiple myeloma. Cancer Res. 2020;80:6711.
-
- Trudel S, McCurdy A, Fu M, Sutherland HJ, Louzada ML, Chu MP, et al. Belantamab Mafodotin in combination with pomalidomide and dexamethasone demonstrates durable responses in triple class exposed/refractory multiple myeloma. Blood. 2022;140:7306–7.
-
- Gavriatopoulou M, Ntanasis-Stathopoulos I, Malandrakis P, Fotiou D, Migkou M, Theodorakakou F, et al. Belantamab mafodotin, lenalidomide, and dexamethasone in transplant-ineligible patients with newly diagnosed multiple myeloma: Analysis of belantamab mafodotin-associated ocular adverse events and their impact on daily functioning from the part 1 of a phase 1/2 study. Am J Hematol. 2024;99:502–4. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

