Upregulation of cholinergic modulators Lypd6 and Lypd6b associated with autism drives anxiety and cognitive decline
- PMID: 39433742
- PMCID: PMC11494011
- DOI: 10.1038/s41420-024-02211-z
Upregulation of cholinergic modulators Lypd6 and Lypd6b associated with autism drives anxiety and cognitive decline
Abstract
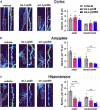
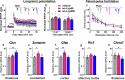
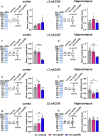
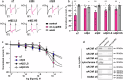
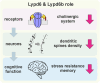
Intellectual disability and autistic features are associated with chromosome region 2q23.q23.2 duplication carrying LYPD6 and LYPD6B genes. Here, we analyzed LYPD6 and LYPD6B expression in patients with different neuropsychiatric disorders. Increased LYPD6 and LYPD6B expression was revealed in autism and other disorders. To study possible consequences of Lypd6 and Lypd6b overexpression in the brain, we used a mouse model with intracerebroventricular delivery of recombinant analogs of these proteins. A two-week infusion evoked significant memory impairment and acute stress. Both modulators downregulated hippocampal and amygdala dendritic spine density. No changes in synaptic plasticity were observed. Intracerebroventricular administration by both proteins downregulated hippocampal expression of Lypd6, Lypd6b, and α7 nicotinic acetylcholine receptor (nAChR). Similar to Lypd6, Lypd6b targeted different nAChR subtypes in the brain with preferential inhibition of α7- and α4β2-nAChRs. Thus, increased Lypd6 and Lypd6b level in the brain are linked to cholinergic system depression, neuronal atrophy, memory decline, and anxiety.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Terry AV, Jones K, Bertrand D. Nicotinic acetylcholine receptors in neurological and psychiatric diseases. Pharmacol Res. 2023;191:106764. - PubMed
-
- Mineur YS, Soares AR, Etherington IM, Abdulla ZI, Picciotto MR. Pathophysiology of nAChRs: limbic circuits and related disorders. Pharmacol Res. 2023;191:106745. - PubMed
-
- Thomsen MS, Weyn A, Mikkelsen JD. Hippocampal α7 nicotinic acetylcholine receptor levels in patients with schizophrenia, bipolar disorder, or major depressive disorder. Bipolar Disord. 2011;13:701–7. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

