Home-based transcranial direct current stimulation treatment for major depressive disorder: a fully remote phase 2 randomized sham-controlled trial
- PMID: 39433921
- PMCID: PMC11750699
- DOI: 10.1038/s41591-024-03305-y
Home-based transcranial direct current stimulation treatment for major depressive disorder: a fully remote phase 2 randomized sham-controlled trial
Abstract
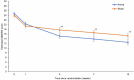
Transcranial direct current stimulation (tDCS) has been proposed as a new treatment in major depressive disorder (MDD). This is a fully remote, multisite, double-blind, placebo-controlled, randomized superiority trial of 10-week home-based tDCS in MDD. Participants were 18 years or older, with MDD in current depressive episode of at least moderate severity as measured using the Hamilton Depression Rating Scale (mean = 19.07 ± 2.73). A total of 174 participants (120 women, 54 men) were randomized to active (n = 87, mean age = 37.09 ± 11.14 years) or sham (n = 87, mean age = 38.32 ± 10.92 years) treatment. tDCS consisted of five sessions per week for 3 weeks then three sessions per week for 7 weeks in a 10-week trial, followed by a 10-week open-label phase. Each session lasted 30 min; the anode was placed over the left dorsolateral prefrontal cortex and the cathode over the right dorsolateral prefrontal cortex (active tDCS 2 mA and sham tDCS 0 mA, with brief ramp up and down to mimic active stimulation). As the primary outcome, depressive symptoms showed significant improvement when measured using the Hamilton Depression Rating Scale: active 9.41 ± 6.25 point improvement (10-week mean = 9.58 ± 6.02) and sham 7.14 ± 6.10 point improvement (10-week mean = 11.66 ± 5.96) (95% confidence interval = 0.51-4.01, P = 0.012). There were no differences in discontinuation rates. In summary, a 10-week home-based tDCS treatment with remote supervision in MDD showed high efficacy, acceptability and safety. ClinicalTrials.gov registration: NCT05202119.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: C.H.Y.F. reports the following competing interests: research grant funding on behalf of the University of East London from Flow Neuroscience (no. R102696); research grant funding from NIMH (no. R01MH134236), the Baszucki Brain Research Fund Milken Institute (no. BD0000009), the Rosetrees Trust (no. CF20212104), the International Psychoanalytic Society (no. 158102845), the MRC (no. G0802594), NARSAD and the Wellcome Trust. She is Associate Editor of Psychoradiology and Section Editor of the Brain Research Bulletin. A.H.Y. reports the following competing interests: paid lectures and advisory boards for the following companies with therapies used in affective and related disorders: Flow Neuroscience, Novartis, Roche, Janssen, Takeda, Noema Pharma, Compass, AstraZeneca, Boehringer Ingelheim, Eli Lilly, LivaNova, Lundbeck, Sunovion, Servier, LivaNova, Janssen, Allegan, Bionomics, Sumitomo Dainippon Pharma, Sage, Novartis and Neurocentrx. He is principal investigator for the following studies: the Restore-Life VNS registry study funded by LivaNova; ESKETINTRD3004: ‘An Open-label, Long-term, Safety and Efficacy Study of Intranasal Esketamine in Treatment-resistant Depression’; The Effects of Psilocybin on Cognitive Function in Healthy Participants; The Safety and Efficacy of Psilocybin in Participants with Treatment-Resistant Depression (P-TRD); A Double-Blind, Randomized, Parallel-Group Study with Quetiapine Extended Release as Comparator to Evaluate the Efficacy and Safety of Seltorexant 20 mg as Adjunctive Therapy to Antidepressants in Adult and Elderly Patients with Major Depressive Disorder with Insomnia Symptoms Who Have Responded Inadequately to Antidepressant Therapy (Janssen); An Open-label, Long-term, Safety and Efficacy Study of Aticaprant as Adjunctive Therapy in Adult and Elderly Participants with Major Depressive Disorder (MDD) (Janssen); A Randomized, Double-blind, Multicentre, Parallel-group, Placebo-controlled Study to Evaluate the Efficacy, Safety, and Tolerability of Aticaprant 10 mg as Adjunctive Therapy in Adult Participants with Major Depressive Disorder (MDD) with Moderate-to-severe Anhedonia and Inadequate Response to Current Antidepressant Therapy; A Study of Disease Characteristics and Real-life Standard of Care Effectiveness in Patients with Major Depressive Disorder (MDD) With Anhedonia and Inadequate Response to Current Antidepressant Therapy Including an SSRI or SNR (Janssen). He is UK Chief Investigator for the following studies: Novartis MDD study no. MIJ821A12201; Compass; and the COMP006 and COMP007 studies. Grant funding (past and present) includes: NIMH (USA); CIHR (Canada); NARSAD (USA); the Stanley Medical Research Institute (USA); MRC (UK); the Wellcome Trust (UK); the Royal College of Physicians of Edinburgh; the British Medical Association (UK); the VGH & UBC Foundation (Canada); WEDC (Canada); the CCS Depression Research Fund (Canada); the Michael Smith Foundation for Health Research (Canada); NIHR (UK). Janssen (UK) and EU Horizon 2020. He is the Editor of the Journal of Psychopharmacology and Deputy Editor of BJPsych Open. He has no shareholdings in pharmaceutical companies. S.S. reports the following competing interests: research grant funding on behalf of the University of Texas Health Science Center at Houston from Flow Neuroscience; paid advisory boards for the following companies: Worldwide Clinical Trials and Inversago; and Vicore Pharma. He is a full-time employee of Intra-Cellular Therapies. He has received grants and research support from NIMH (USA) (no. 1R21MH119441-01A1), NIMH (no. 1R21MH129888-01A1), NICHD (no. 1R21HD106779-01A1), SAMHSA (no. 6H79FG000470-01M003) and Fizer foundation. He has received research funding as a principal investigator or study/subinvestigator from or participated as consultant/speaker for Flow Neuroscience, COMPASS Pathways, LivaNova, Janssen, Relmada and the Psychiatry Education Forum. Intra-Cellular Therapies or National Institutes of Health (NIH) or SAMHSA or any other organizations had no role in study design and conduct; the collection, management, analysis and interpretation of the data; the preparation, review or approval of the manuscript; and the decision to submit the manuscript for publication. The study’s content is solely the responsibility of the authors and does not necessarily represent the official views of the Intra-Cellular Therapies or NIH or SAMHSA. R.M-V. has received consulting fees from Eurofarma Pharmaceuticals, Abbott and BioStrategies group; has research contracts with Boerhinger Ingelheim and Janssen Pharmaceuticals; and has received speaker fees from Otsuka, EMS and Cristalia. He is a member of the scientific boards of Symbinas Pharmaceuticals and Allergan. He is also the principal investigator for the following grants: NIH (nos. R21HD106779 and R21MH129888), Milken Institute (no. BD-0000000081). D.M. and L.H. work for Biomedical Statistical Consulting; they provide statistical support to MCRA and received payments from Flow Neuroscience. A.-R.G.-N., G.S., H.H., J.C.S., M.R., N.L., P.J.L., P.O., R.D.W. and S.S.K. declare no competing interests.
Figures



References
-
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates (2017); https://apps.who.int/iris/bitstream/handle/10665/254610/WHO-MSD-MER-2017...
-
- Cuijpers, P. et al. The effects of psychotherapies for major depression in adults on remission, recovery and improvement: a meta-analysis. J. Affect. Disord.159, 118–126 (2014). - PubMed
-
- Rush, A. J. et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am. J. Psychiatry163, 1905–1917 (2006). - PubMed
-
- Woodham, R., Rimmer, R. M., Mutz, J. & Fu, C. H. Y. Is tDCS a potential first line treatment for major depression? Int. Rev. Psychiatry33, 250–265 (2021). - PubMed
-
- Creutzfeldt, O. D., Fromm, G. H. & Kapp, H. Influence of transcortical d-c currents on cortical neuronal activity. Exp. Neurol.5, 436–452 (1962). - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

