Spatial-transcriptomic profiling: a new lens for understanding myelofibrosis pathophysiology
- PMID: 39434124
- PMCID: PMC11492555
- DOI: 10.1186/s12964-024-01877-3
Spatial-transcriptomic profiling: a new lens for understanding myelofibrosis pathophysiology
Abstract
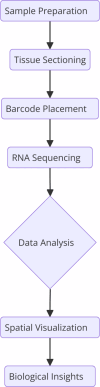
Myelofibrosis (MF) is a complex myeloproliferative neoplasm characterized by abnormal hematopoietic stem cell proliferation and subsequent bone marrow (BM) fibrosis. First documented in the late 19th century, MF has since been extensively studied to unravel its pathophysiology, clinical phenotypes, and therapeutic interventions. MF can be classified into primary and secondary forms, both driven by mutations in genes such as JAK2, CALR, and MPL, which activate the JAK-STAT signaling pathway. These driver mutations are frequently accompanied by additional non-driver mutations in genes like TET2, SRSF2, and TP53, contributing to disease complexity. The BM microenvironment, consisting of stromal cells, extracellular matrix, and cytokines such as TGF-β and TNF-α, plays a critical role in fibrosis and aberrant hematopoiesis. Clinically, MF manifests with symptoms ranging from anemia, splenomegaly, and fatigue to severe complications such as leukemic transformation. Splenomegaly, caused by extramedullary hematopoiesis, leads to abdominal discomfort and early satiety. Current therapeutic strategies include JAK inhibitors like Ruxolitinib, which target the JAK-STAT pathway, alongside supportive treatments such as blood transfusions, erythropoiesis-stimulating agents and developing combinatorial approaches. Allogeneic hematopoietic stem cell transplantation remains the only curative option, though it is limited to younger, high-risk patients. Recently approved JAK inhibitors, including Fedratinib, Pacritinib, and Momelotinib, have expanded the therapeutic landscape. Spatially Resolved Transcriptomics (SRT) has revolutionized the study of gene expression within the spatial context of tissues, providing unprecedented insights into cellular heterogeneity, spatial gene regulation, and microenvironmental interactions, including stromal-hematopoietic dynamics. SRT enables high-resolution mapping of gene expression in the BM and spleen, revealing molecular signatures, spatial heterogeneity, and pathological niches that drive disease progression. These technologies elucidate the role of the spleen in MF, highlighting its transformation into a site of abnormal hematopoietic activity, fibrotic changes, and immune cell infiltration, functioning as a "tumor surrogate." By profiling diverse cell populations and molecular alterations within the BM and spleen, SRT facilitates a deeper understanding of MF pathophysiology, helping identify novel therapeutic targets and biomarkers. Ultimately, integrating spatial transcriptomics into MF research promises to enhance diagnostic precision and therapeutic innovation, addressing the multifaceted challenges of this disease.
Keywords: Jak inhibitors; Myelofibrosis; Myeloproliferative neoplasms; Personalized medicine; Spatial transcriptomics; Spleen.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Bertozzi I, et al. Thrombotic risk correlates with mutational status in true essential thrombocythemia. Eur J Clin Invest. 2016;46:683–9. - PubMed
-
- Heuck G. Zwei Falle Von Leukamie Mit Eigenthumlichem Blut- resp. Knochenmarksbefund. (two cases of leukemia with peculiar blood and BM findings, respectively). Arch Pathol Anat Physiol Virchows. 1879;78:475–96.
-
- Yogarajah M, Tefferi A. Leukemic Transformation in Myeloproliferative neoplasms: a literature review on risk, characteristics, and Outcome. Mayo Clin Proc. 2017;92:1118–28. - PubMed
-
- Polverelli N, et al. Splenomegaly in patients with primary or secondary myelofibrosis who are candidates for allogeneic hematopoietic cell transplantation: a position paper on behalf of the Chronic Malignancies Working Party of the EBMT. Lancet Haematol. 2023;10:e59–70. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

