Albumin reprograms the B cell transcriptional landscape and improves neutrophil antimicrobial function in patients with decompensated cirrhosis
- PMID: 39434891
- PMCID: PMC11490805
- DOI: 10.1016/j.jhepr.2024.101184
Albumin reprograms the B cell transcriptional landscape and improves neutrophil antimicrobial function in patients with decompensated cirrhosis
Abstract
Background & aims: Patients with acutely decompensated (AD) cirrhosis are immunocompromised and particularly susceptible to infections. This study investigated the immunomodulatory actions of albumin by which this protein may lower the incidence of infections.
Methods: Blood immunophenotyping was performed in 11 patients with AD cirrhosis and 10 healthy volunteers (HV). Bulk and single-cell RNA sequencing (scRNA-seq) and flow cytometry were performed in peripheral blood mononuclear cells (PBMCs) from 20 patients with AD cirrhosis and 34 HV exposed to albumin. Albumin's effects on degranulation, phagocytosis, chemotaxis, and swarming of neutrophils from six patients with AD cirrhosis and nine HV were assessed by measuring myeloperoxidase enzymatic activity, the engulfment of fluorescent-labeled Escherichia coli and zymosan, and interactions of neutrophils with Candida albicans at single-cell resolution in microfluidic chambers, respectively. Whole blood RNA sequencing (RNA-seq) analyses were performed in 49 patients admitted for severe AD cirrhosis, of whom 30 received albumin during hospitalization.
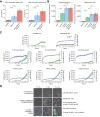
Results: Compared with HV, patients with AD cirrhosis showed severe lymphopenia and defective neutrophil antimicrobial function. Bulk and scRNA-seq analyses revealed significantly (false discovery rate [FDR] <0.05) increased signatures related to B cells, myeloid cells, and CD4+ T cells in PBMCs incubated with albumin. Changes in the B cell population were confirmed by flow cytometry. Neutrophils exposed to albumin also exhibited augmented chemotactic and degranulation responses, enhanced phagocytosis, and increased pathogen-restrictive swarming. RNA-seq data analysis in patients who had received albumin revealed specific upregulation of signatures related to B cells and neutrophils together with transcriptional changes in CD4+ T cells (FDR <0.05).
Conclusions: The finding that albumin promotes the transcriptional reprogramming and expansion of the B cell compartment and improves neutrophil antimicrobial functions indicates mechanisms that may lower the incidence of infections in patients with severe AD cirrhosis receiving albumin therapy.
Impact and implications: Patients with acutely decompensated cirrhosis receiving albumin as treatment have a lower incidence of infections. The reason for this protection is currently unknown, but the present study provides data that support the ability of albumin to boost the antimicrobial functions of immune cells in these patients. Moreover, these findings encourage the design of controlled clinical studies specifically aimed at investigating the effects of albumin administration on the immune system.
Keywords: Gene expression; Immunosuppression; Inflammation; Multiorgan failure.
© 2024 The Author(s).
Figures




References
-
- Moreau R., Jalan R., Ginès P., et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–1437. - PubMed
-
- Arroyo V., Moreau R., Jalan R. Acute-on-chronic liver failure. N Engl J Med. 2020;382:2137–2145. - PubMed
-
- Clària J., Stauber R.E., Coenraad M.J., et al. Systemic inflammation in decompensated cirrhosis: characterization and role in acute-on-chronic liver failure. Hepatology. 2016;64:1249–1264. - PubMed
-
- López-Vicario C., Checa A., Urdangarin A., et al. Targeted lipidomics reveals extensive changes in circulating lipid mediators in patients with acutely decompensated cirrhosis. J Hepatol. 2020;73:817–828. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

