Tumor volume features predict survival outcomes for patients diagnosed with diffuse intrinsic pontine glioma
- PMID: 39434924
- PMCID: PMC11492488
- DOI: 10.1093/noajnl/vdae151
Tumor volume features predict survival outcomes for patients diagnosed with diffuse intrinsic pontine glioma
Abstract
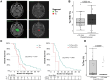
Background: Diffuse intrinsic pontine glioma (DIPG) is a fatal childhood central nervous system tumor. Diagnosis and monitoring of tumor response to therapy is based on magnetic resonance imaging (MRI). MRI-based analyses of tumor volume and appearance may aid in the prediction of patient overall survival (OS).
Methods: Contrast-enhanced T1- and FLAIR/T2-weighted MR images were retrospectively collected from children with classical DIPG diagnosed by imaging (n = 43 patients). MRI features were evaluated at diagnosis (n = 43 patients) and post-radiation (n = 40 patients) to determine OS outcome predictors. Features included 3D tumor volume (Twv), contrast-enhancing tumor core volume (Tc), Tc relative to Twv (TC/Twv), and Twv relative to whole brain volume. Support vector machine (SVM) learning was used to identify feature combinations that predicted OS outcome (defined as OS shorter or longer than 12 months from diagnosis).
Results: Features associated with poor OS outcome included the presence of contrast-enhancing tumor at diagnosis, >15% Tc/Twv post-radiation therapy (RT), and >20% ∆Tc/Twv post-RT. Consistently, SVM learning identified Tc/Twv at diagnosis (prediction accuracy of 74%) and ∆Tc/Twv at <2 months post-RT (accuracy = 75%) as primary features of poor survival.
Conclusions: This study demonstrates that tumor imaging features at diagnosis and within 4 months of RT can predict differential OS outcomes in DIPG. These findings provide a framework for incorporating tumor volume-based predictive analyses into the clinical setting, with the potential for treatment customization based on tumor risk characteristics and future applications of machine-learning-based analysis.
Keywords: MRI; SVM learning; diffuse intrinsic pontine glioma; survival outcomes; tumor volume.
© The Author(s) 2024. Published by Oxford University Press, the Society for Neuro-Oncology and the European Association of Neuro-Oncology.
Conflict of interest statement
None of the authors have a conflict to declare related to the work in this manuscript.
Figures


References
-
- Hoffman LM, Veldhuijzen van Zanten SEM, Colditz N, et al.. Clinical, radiologic, pathologic, and molecular characteristics of long-term survivors of diffuse intrinsic pontine glioma (DIPG): a collaborative report from the International and European Society for Pediatric Oncology DIPG Registries. J Clin Oncol. 2018;36(19):1963–1972. - PMC - PubMed
-
- Giagnacovo M, Antonelli M, Biassoni V, et al.. Retrospective analysis on the consistency of MRI features with histological and molecular markers in diffuse intrinsic pontine glioma (DIPG). Child’s Nerv Syst. 2020;36(4):697–704. - PubMed
LinkOut - more resources
Full Text Sources
