Regorafenib promotes antitumor progression in melanoma by reducing RRM2
- PMID: 39435141
- PMCID: PMC11492136
- DOI: 10.1016/j.isci.2024.110993
Regorafenib promotes antitumor progression in melanoma by reducing RRM2
Abstract
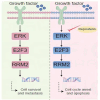
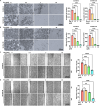
Melanoma is a malignant tumor with a terrible prognosis. Although so many therapies are used for melanoma, the overall survival rate is still poor globally. Novel therapies are still required. In our study, the role and potential mechanism of regorafenib in melanoma are explored. Regorafenib has the ability to limit the growth, invasion, and metastasis of melanoma cells but to upregulate apoptosis-prompting markers (cleaved-PARP and Bax). RRM2 is identified to be the downstream target of regorafenib by RNA sequencing. In addition, we discovered that RRM2 inhibition and regorafenib have comparable effects on melanoma cells. Rescue experiments showed that RRM2 is crucial in regulating regorafenib's anti-melanoma progression. Moreover, ERK/E2F3 signaling influences regorafenib's ability to suppress melanoma cell growth. Ultimately, regorafenib significantly inhibits tumor growth in vivo. In conclusion, our finding demonstrated that regorafenib promotes antitumor progression in melanoma by reducing RRM2.
Keywords: Biological sciences; Cancer; Therapy.
© 2024 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Swetter S.M., Tsao H., Bichakjian C.K., Curiel-Lewandrowski C., Elder D.E., Gershenwald J.E., Guild V., Grant-Kels J.M., Halpern A.C., Johnson T.M., et al. Guidelines of care for the management of primary cutaneous melanoma. J. Am. Acad. Dermatol. 2019;80:208–250. doi: 10.1016/j.jaad.2018.08.055. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

