Enhanced Wound Healing With β-Chitosan-Zinc Oxide Nanoparticles: Insights From Zebrafish Models
- PMID: 39435246
- PMCID: PMC11493322
- DOI: 10.7759/cureus.69861
Enhanced Wound Healing With β-Chitosan-Zinc Oxide Nanoparticles: Insights From Zebrafish Models
Abstract
Introduction: Wound healing is a complex physiological process essential for the restoration of tissue integrity and function. Novel therapeutic approaches are urgently needed to enhance wound-healing outcomes. Nanotechnology, particularly zinc oxide nanoparticles, has shown promise due to its antimicrobial, anti-inflammatory, and regenerative properties. β-chitosan, derived from squid pens, possesses superior solubility and bioactivity compared to α-chitosan, making it a valuable biomaterial for biomedical applications. Through the integration of β-chitosan and zinc oxide nanoparticles, this study seeks to use the complementary properties of both substances to overcome present constraints in wound care treatments.
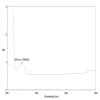
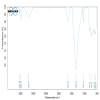
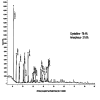
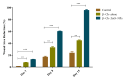
Methods: β-chitosan was extracted from squid pens and characterized for its molecular weight, degree of deacetylation, and solubility properties. Further characterization of the synthesized zinc oxide nanoparticles involved Fourier transform infrared spectroscopy to analyze chemical bonding and functional groups, ultraviolet-visible spectroscopy to determine optional properties such as band gap energy, X-ray diffraction spectroscopy to confirm the crystalline phase and calculate crystallite size, and the size was confirmed with the scanning electron microscope. Each technique provided complementary information, ensuring a comprehensive understanding of the synthesized nanoparticles' properties and their potential applications. Adult zebrafish (six to eight months old) were employed as a model organism due to their genetic similarity to humans and regenerative capabilities. Zebrafish were wounded and divided into treatment and control groups, with β-chitosan and β-chitosan-derived zinc nanoparticles treatments administrated at 50 µg/ml, while control groups received 0.05% phosphate buffer saline. The treatments, conducted in triplicate, enabled a comparative analysis of wound closure activity between β-chitosan-derived zinc nanoparticles' healing effects against standard and baseline treatments. Further, gene expression analysis on Bax, BCl-2, IL-2, IL-6, and tumor necrosis factor-alpha (TNF-a) was done using reverse transcriptase polymerase chain reaction.
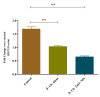
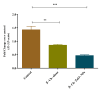
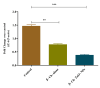
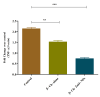
Results: Characterization studies confirmed the successful synthesis of β-chitosan-derived zinc oxide nanoparticles and a crystalline structure corresponding to zinc oxide. Treatment with β-chitosan-derived zinc oxide nanoparticles significantly accelerated wound closure compared to controls and other treatment groups. Microscopic analysis demonstrated enhanced epithelialization, reduced inflammatory cell infiltration, increased collagen deposition, and improved tissue organization in wounds treated with β-chitosan-derived zinc oxide nanoparticles. Gene expression analysis revealed downregulation of inflammation-causing genes such as BCl-2, IL-2, IL-6, and TNF-a, hence it showed wound-healing activity. The results were statistically significant (p < 0.05).
Conclusion: β-chitosan-derived zinc oxide nanoparticles show promising potential as a novel therapeutic strategy for enhancing wound healing. The synergistic effects of β-chitosan and zinc oxide nanoparticles address multiple aspects of wound healing, including antimicrobial activity, inflammation modulation, and tissue regeneration. This study highlights the advantages of nanotechnology in wound care and underscores the need for further research to optimize nanoparticle formulations for clinical applications.
Keywords: antimicrobial; nanotechnology; regenerative properties; wound healing; zebrafish; zinc oxide nanoparticles; β-chitosan.
Copyright © 2024, Ramachandran et al.
Conflict of interest statement
Human subjects: All authors have confirmed that this study did not involve human participants or tissue. Animal subjects: Biomedical Research Unit and Lab Animal Centre (BRULAC), Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences (SIMATS) Issued protocol number BRULAC/SDCH/SIMATS/IAEC/06-2023/15. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures



References
-
- Anti-angiogenic and anti-metastatic effects of biogenic silver nanoparticles synthesized using Azadirachta indica. Kitimu S, Kirira P, Abdille A, et al. Adv Biosci Biotechnol. 2022;13:188–206.
-
- Inflammation: wound healing in zebrafish. Martin P, Feng Y. Nature. 2009;459:921–923. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
