RUNX2 as a novel biomarker for early identification of patients progressing to advanced-stage mycosis fungoides
- PMID: 39435287
- PMCID: PMC11491341
- DOI: 10.3389/fonc.2024.1421443
RUNX2 as a novel biomarker for early identification of patients progressing to advanced-stage mycosis fungoides
Abstract
Introduction: The majority of patients with mycosis fungoides (MF) have an indolent disease course, but a substantial fraction (20-30%) of patients progress to advanced stages - usually with a grave prognosis. Early differentiation between indolent and aggressive types of MF is important for the choice of treatment regimen and monitoring of the individual patient. Good biomarkers are therefore desired.
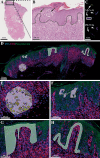
Methods: Here, we used spatial transcriptomics on skin samples at time-of-diagnosis to enable prediction of patients who later progressed to advanced stages of MF. Formalin-fixed, paraffin-embedded skin biopsies at time of diagnosis from six patients with MF who progressed to advanced stages of disease within 4 months to 12 years after diagnosis, and nine patients who remained in early-stage disease over 9 to 27 years were analyzed using the GeoMx Digital Spatial Profiler to capture spatially resolved high-plex RNA gene expression data. Five different regions of interest (the epidermis, the basal layer of epidermis, CD4+ T-cells and neighboring cells, and Pautrier's microabscesses) were profiled for further assessment.
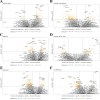
Results and discussion: Interestingly, RUNX2, SHMT2, and MCM7 were upregulated in the enriched population of malignant T-cells in Pautrier's microabscesses in patients who later developed advanced stages of disease. Expression of RUNX2, SHMT2 and MCM7 in malignant T-cells was confirmed in a subset of patients in MF skin using scRNA-seq datasets across multiple studies and correlating with stage of disease. Taken together, we provide first evidence that RUNX2 has potential as a biomarker to identify MF patients progressing to advanced stage disease. As RUNX2 has not previously been linked to MF, our data also shows the analytical strength of combining spatial transcriptomics with scRNA-seq analysis.
Keywords: RNA profiling; biomarkers; digital spatial profiling; mycosis fungoides; spatial transcriptomics.
Copyright © 2024 Danielsen, Emmanuel, Nielsen, Lindahl, Gluud, Ødum, Raaby, Steiniche, Iversen, Bech, Buus and Johansen.
Conflict of interest statement
Author TE has participated in investigator-initiated clinical trials sponsored by Janssen and Leo Pharma and has served as paid speaker for BMS. Author CJ has served as a consultant and/or paid speaker for Eli Lilly, Abbvie, Leo Pharma and L’Oréal. Author LI has served as a consultant and/or paid speaker for and/or participated in clinical trials sponsored by: AbbVie, Almirall, Amgen, AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly, Janssen Cilag, Kyowa, Leo Pharma, MSD, Novartis, Pfizer, Regranion, Samsung, Union Therapeutics and UCB. Author LI was employed by the company MC2 Therapeutics A/S. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

