Three-dimensional view of microglia-amyloid plaque interactions
- PMID: 39435610
- PMCID: PMC11660520
- DOI: 10.1002/glia.24628
Three-dimensional view of microglia-amyloid plaque interactions
Abstract
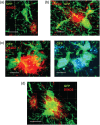
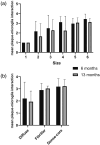
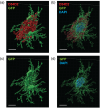
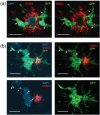
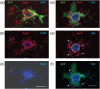
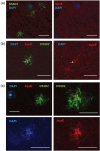
Recent gene expression studies have revealed about 10 different states of microglia, some of which are characteristic for Alzheimer-like amyloid plaque pathology. However, it is not presently known how these translate into morphological features that would reflect microglia interaction with amyloid plaques. With optimized conditions for confocal microscopy in amyloid plaque forming APP/PS1 transgenic mice we reveal new details of how microglia processes interact with amyloid plaques. The microglia contacts differed drastically between purely diffuse plaque and those with a fibrillar core. We identified microglia that extend their enlarged processes through the diffuse shell of the amyloid plaques and cover the fibrillar plaque core with snowplow-like expanded end-feet. These end-feet were filled with the lysosomal marker CD68, while both non-fibrillar and fibrillar amyloid was found in perinuclear vesicles of some "snowplower" microglia. In the organized dense-core plaques, we consistently saw a layer of Apolipoprotein E (ApoE) between the fibrillar core and the microglial end-feet. ApoE covered also loose fibrillar amyloid and diffuse amyloid plaques that were about 10 μm or larger in diameter. These findings are compatible with both amyloid plaque phagocytosis and compaction by microglia. Further, they support a chemotactic role of ApoE for microglia contacts with amyloid plaques.
Keywords: 3D rendering; ApoE; confocal imaging; dense‐core plaque; transgenic mice.
© 2024 The Author(s). GLIA published by Wiley Periodicals LLC.
Figures



References
-
- Bales, K. R. , Verina, T. , Dodel, R. C. , Du, Y. , Altstiel, L. , Bender, M. , Hyslop, P. , Johnstone, E. M. , Little, S. P. , Cummins, D. J. , Piccardo, P. , Ghetti, B. , & Paul, S. M. (1997). Lack of apolipoprotein E dramatically reduces amyloid beta‐peptide deposition. Nature Genetics, 17(3), 263–264. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

