The biology of water homeostasis
- PMID: 39435642
- PMCID: PMC11960738
- DOI: 10.1093/ndt/gfae235
The biology of water homeostasis
Abstract
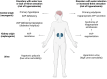
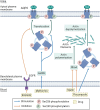
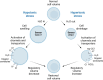
Water homeostasis is controlled by a brain-kidney axis that consists of central osmoreceptors, synthesis and secretion of arginine vasopressin (AVP) and AVP-responsive aquaporin-2 (AQP2) water channels in kidney collecting duct principal cells that facilitate water reabsorption. In addition to AVP, thirst represents a second line of defence to maintain water balance. Water balance disorders arise because of deficiency, resistance or inappropriate secretion of AVP or disturbances in thirst sensation (hypodipsia, polydipsia). People with water balance disorders are prone to develop hyponatraemia or hypernatraemia, which expose cells to osmotic stress and activate cell volume regulation mechanisms. This review covers several recent insights that have expanded our understanding of central osmoregulation, AQP2 regulation and cell volume regulation. This includes the role of with no lysine kinase 1 (WNK1) as a putative central osmolality sensor and, more generally, as an intracellular crowding sensor that coordinates the cell volume rescue response by activating sodium and potassium cotransporters. Furthermore, several new regulators of AQP2 have been identified, including AVP-dependent AQP2 regulation (yes-associated protein, nuclear factor of activated T-cells, microRNAs) and AVP-independent AQP2 regulation (epidermal growth factor receptor, fluconazole, prostaglandin E2). It is also becoming increasingly clear that long-term cell volume adaptation to chronic hypotonicity through release of organic osmolytes comes at the expense of compromised organ function. This potentially explains the complications of chronic hyponatraemia, including cognitive impairment, bone loss and vascular calcification. This review illustrates why these new insights derived from basic science are also relevant for developing new approaches to treat water balance disorders.
Keywords: aquaporin-2; cell volume regulation; hypernatraemia; hyponatraemia; vasopressin.
© The Author(s) 2024. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
None declared.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

