Distinct strategies for intravascular triglyceride metabolism in hearts of mammals and lower vertebrate species
- PMID: 39435661
- PMCID: PMC11529983
- DOI: 10.1172/jci.insight.184940
Distinct strategies for intravascular triglyceride metabolism in hearts of mammals and lower vertebrate species
Abstract
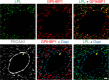
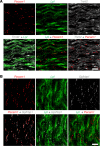
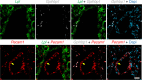
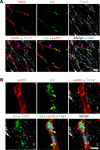
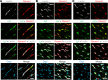
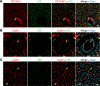
Lipoprotein lipase (LPL) and multiple regulators of LPL activity (e.g., APOC2 and ANGPTL4) are present in all vertebrates, but GPIHBP1-the endothelial cell (EC) protein that captures LPL within the subendothelial spaces and transports it to its site of action in the capillary lumen-is present in mammals but in not chickens or other lower vertebrates. In mammals, GPIHBP1 deficiency causes severe hypertriglyceridemia, but chickens maintain low triglyceride levels despite the absence of GPIHBP1. To understand intravascular lipolysis in lower vertebrates, we examined LPL expression in mouse and chicken hearts. In both species, LPL was abundant on capillaries, but the distribution of Lpl transcripts was strikingly different. In mouse hearts, Lpl transcripts were extremely abundant in cardiomyocytes but were barely detectable in capillary ECs. In chicken hearts, Lpl transcripts were absent in cardiomyocytes but abundant in capillary ECs. In zebrafish hearts, lpl transcripts were also in capillary ECs but not cardiomyocytes. In both mouse and chicken hearts, LPL was present, as judged by immunogold electron microscopy, in the glycocalyx of capillary ECs. Thus, mammals produce LPL in cardiomyocytes and rely on GPIHBP1 to transport the LPL into capillaries, whereas lower vertebrates produce LPL directly in capillary ECs, rendering an LPL transporter unnecessary.
Keywords: Cardiology; Endothelial cells; Lipoproteins; Metabolism.
Conflict of interest statement
Figures






References
-
- Weinstock PH, et al. Severe hypertriglyceridemia, reduced high density lipoprotein, and neonatal death in lipoprotein lipase knockout mice. Mild hypertriglyceridemia with impaired very low density lipoprotein clearance in heterozygotes. J Clin Invest. 1995;96(6):2555–2568. doi: 10.1172/JCI118319. - DOI - PMC - PubMed

