The contribution of neutrophils to bacteriophage clearance and pharmacokinetics in vivo
- PMID: 39435664
- PMCID: PMC11530120
- DOI: 10.1172/jci.insight.181309
The contribution of neutrophils to bacteriophage clearance and pharmacokinetics in vivo
Abstract
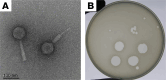
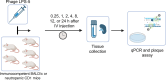
With the increasing prevalence of antimicrobial-resistant bacterial infections, there is interest in using bacteriophages (phages) to treat such infections. However, the factors that govern bacteriophage pharmacokinetics in vivo remain poorly understood. Here, we have examined the contribution of neutrophils, the most abundant phagocytes in the body, to the pharmacokinetics of i.v. administered bacteriophage in uninfected mice. A single dose of LPS-5, a bacteriophage recently used in human clinical trials to treat drug-resistant Pseudomonas aeruginosa, was administered i.v. to both immunocompetent BALB/c and neutropenic CD1 mice. Phage concentrations were assessed in peripheral blood and spleen at 0.25, 1, 2, 4, 8, 12, and 24 hours after administration by plaque assay and qPCR. We observed that the phage clearance was only minimally affected by neutropenia. Indeed, the half-lives of phages in blood in BALB/c and CD1 mice were 3.45 and 3.66 hours, respectively. These data suggest that neutrophil-mediated phagocytosis is not a major determinant of phage clearance. Conversely, we observed a substantial discrepancy in circulating phage levels over time when measured by qPCR versus plaque assay, suggesting that significant inactivation of circulating phages occurs over time. These data indicate that alternative factors, but not neutrophils, inactivate i.v. administered phages.
Keywords: Bacterial infections; Infectious disease.
Figures

Update of
-
The contribution of neutrophils to bacteriophage clearance and pharmacokinetics in vivo.bioRxiv [Preprint]. 2024 Jan 27:2024.01.25.577154. doi: 10.1101/2024.01.25.577154. bioRxiv. 2024. Update in: JCI Insight. 2024 Oct 22;9(20):e181309. doi: 10.1172/jci.insight.181309. PMID: 38328123 Free PMC article. Updated. Preprint.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

