Light induces a rapid increase in cAMP and activates PKA in rod outer segments of the frog retina
- PMID: 39436404
- PMCID: PMC11498274
- DOI: 10.1085/jgp.202313530
Light induces a rapid increase in cAMP and activates PKA in rod outer segments of the frog retina
Abstract
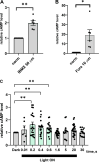
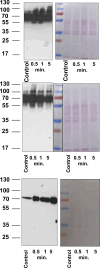
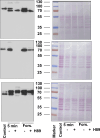
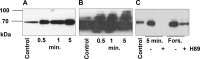
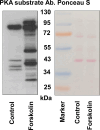
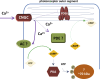
The phototransduction cascade enables the photoreceptor to detect light over a wide range of intensities without saturation. The main second messenger of the cascade is cGMP and the primary regulatory mechanism is calcium feedback. However, some experimental data suggest that cAMP may also play a role in regulating the phototransduction cascade, but this would require changes in cAMP on a time scale of seconds. Currently, there is a lack of data on the dynamics of changes in intracellular cAMP levels on this timescale. This is largely due to the specificity of the sensory modality of photoreceptors, which makes it practically impossible to use conventional experimental approaches based on fluorescence methods. In this study, we employed the method of rapid cryofixation of retinal samples after light stimulation and subsequent isolation of outer segment preparations. The study employed highly sensitive metabolomics approaches to measure levels of cAMP. Additionally, PKA activity was measured in the samples using a western blot. The results indicate that when exposed to near-saturating but still moderate light, cAMP levels increase transiently within the first second and then return to pre-stimulus levels. The increase in cAMP activates PKA, resulting in the phosphorylation of PKA-specific substrates in frog retinal outer segments.
© 2024 Chernyshkova et al.
Conflict of interest statement
Disclosures: D. Meshalkina reported grants from the Russian Ministry of Science and Education project 075-15-2022-296 World-class research center Pavlov Center “Integrative Physiology to Medicine, High-Tech Healthcare and Technologies of Stress Resistance” outside the submitted work. No other disclosures were reported.
Figures

References
-
- Astakhova, L.A., Kapitskii S.V., Govardovskii V.I., and Firsov M.L.. 2014. Cyclic AMP as a regulator of the phototransduction cascade. Neurosci. Behav. Physiol. 44:664–671. 10.1007/s11055-014-9967-5 - DOI
-
- Beck, F., Geiger J., Gambaryan S., Veit J., Vaudel M., Nollau P., Kohlbacher O., Martens L., Walter U., Sickmann A., and Zahedi R.P.. 2014. Time-resolved characterization of cAMP/PKA-dependent signaling reveals that platelet inhibition is a concerted process involving multiple signaling pathways. Blood. 123:e1–e10. 10.1182/blood-2013-07-512384 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

