Non-allergic Hypersensitivity Reactions to Immunoglobulin Preparations in Antibody Deficiencies: What Role for Anti-IgA IgG and Complement Activation?
- PMID: 39436576
- PMCID: PMC11638320
- DOI: 10.1007/s12016-024-09007-0
Non-allergic Hypersensitivity Reactions to Immunoglobulin Preparations in Antibody Deficiencies: What Role for Anti-IgA IgG and Complement Activation?
Abstract
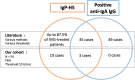
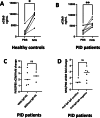
The presence of IgG anti-IgA in the serum of primary immunodeficiency (PID) patients has long been considered responsible for hypersensitivity (HS) to immunoglobulin preparations (IgPs), but this link is increasingly being questioned. The aim of this work was to describe the prevalence of IgG anti-IgA and its association with HS, and to explore a new pathophysiological hypothesis involving the complement system. We measured IgG anti-IgA, using a standardised commercial technique, in controls and PID patients, and compared our results to a systematic literature review. We measured complement activation in PID patients before and after IgP infusion, and in vitro after incubation of IgP with serum from controls and PID patients. IgG anti-IgA was detected in 6% (n = 2/32) of PID patients, 30% (n = 3/10) of selective IgA deficiency patients and 2% (n = 1/46) of healthy controls. In the literature and our study, 38 PID patients had IgG anti-IgA and HS to IgPs and 9 had IgG anti-IgA but good tolerance to IgPs. In our patients, we observed a constant complement activation after IgP infusion compared to baseline. In vitro, IgP induced significant complement activation with all sera from tested individuals. IgA immunisation is not rare in PID, higher in selective IgA deficiency, but may also occur in healthy controls. Our results question the clinical relevance and pathophysiological implication of IgG anti-IgA in the context of HS with IgPs. Complement activation-related pseudoallergy could explain the clinical characteristics and natural history of HS symptoms.
Keywords: Anaphylaxis; Anti-IgA IgG; CARPA; Complement; Intravenous immunoglobulins; Primary immunodeficiency.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics Approval: In line with French recommendations, no ethical approval was requested for this non-interventional study (MR-004 reference methodology). The data and the patients’ sample collections were declared to the CNIL (DEC24-089). Consent to Participate: In line with the regulations laid down by the French National Data Protection Commission (CNIL) and international guidelines, written, informed consent was neither required nor requested for this non-interventional study. The patients received by mail an information letter about the study and a non-opposition notice, and had the opportunity to refuse their inclusion. Consent for Publication: No identifying individual participants’ data are included in this manuscript. Competing Interests: Guillaume Lefèvre has received honoraria for serving on the board and for participating in symposia from Takeda, LFB, Griffols, research support from LFB, Takeda, CSL Behring, Biotest, Octapharma. David Launay has received honoraria for board or symposia from Takeda, Biocryst, Astra-Zeneca and CSL Behring, and research support from CSL Behring, and Octapharma. Emmanuel Ledoult has received consulting and personal fees from Takeda, Grifols, GSK and Astra-Zeneca.
Figures


References
-
- Bruton OC (1952) Agammaglobulinemia. Pediatrics 9(6):722–728 - PubMed
-
- Williams SJ, Gupta S (2017) Anaphylaxis to IVIG. Arch Immunol Ther Exp 65(1):11–19 - PubMed
-
- Gallagher PE, Buckley RH (1982) Anaphylactic reactions and anti-IgA antibodies in common variable agammaglobulinemia: IgA deficient plasma treatment. J Allergy Clin Immunol 69(1):120
-
- Vyas GN, Perkins HA, Fudenberg HH (1968) Anaphylactoid transfusion reactions associated with anti-IgA. Lancet 2(7563):312–5 - PubMed
-
- Almogren A, Kerr MA (2008) Irreversible aggregation of the Fc fragment derived from polymeric but not monomeric serum IgA1—implications in IgA-mediated disease. Mol Immunol 45(1):87–94 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

