Real-World Outcomes with the KEYNOTE-522 Regimen in Early-Stage Triple-Negative Breast Cancer
- PMID: 39436619
- PMCID: PMC11843215
- DOI: 10.1245/s10434-024-16390-7
Real-World Outcomes with the KEYNOTE-522 Regimen in Early-Stage Triple-Negative Breast Cancer
Abstract
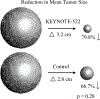
Background: This study aimed to determine if the neoadjuvant (NAT) KEYNOTE-522 regimen was associated with higher rates of pathologic complete response (pCR), corresponding to higher rates of breast conservation therapy (BCT) in early-stage triple-negative breast cancer (TNBC) patients.
Patients and methods: Stage II-III TNBC patients diagnosed between 2019 and 2022 who underwent NAT were analyzed retrospectively. NAT with KEYNOTE-522 versus control NAT were compared for rates of BCT, axillary node dissection (ALND), pCR, and survival outcomes. The prevalence of immune-related adverse events (irAE) from chemoimmunotherapy was recorded.
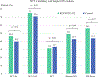
Results: Of 240 patients identified: 86 received KEYNOTE-522 and 154 received control. The frequency of pCR was significantly higher in KEYNOTE versus the control cohort, 59.3% and 33.1%, respectively (p = 0.001). There was no significant difference in the rate of BCT between the control (33.1%) and the KEYNOTE-522 (32.1%) groups (p = 0.47). Rates of ALND were significantly lower with KEYNOTE-522 (25.6%) as compared with control (39.6%); p = 0.03. The rate of development of grade 2 or higher irAEs was 34.9%. At a median follow-up of 2.4 years, there was no difference in survival outcomes. BRCA1 patients had high rates of pCR regardless of treatment group, KEYNOTE-522: 80.0% (4/5) and control: 75% (9/12), (p = 1).
Conclusion: This real-world evidence supports the use of the KEYNOTE-522 regimen in patients with early-stage TNBC given the higher pCR rate and corresponding decrease in the rate of ALND. The majority of patients in both NAT cohorts became BCT eligible, but the rate of BCT did not differ between the two groups.
Keywords: Breast conservation therapy; Immune-related adverse events; Immunotherapy; KEYNOTE-522; Pathologic complete response; Pembrolizumab; Triple-negative breast cancer.
© 2024. Society of Surgical Oncology.
Conflict of interest statement
Disclosure: No commercial interests in the subject of the study to declare. No financial support was provided to declare. Dr. Lang received honoraria from a Novartis advisory board. This project was presented in part as a poster presentation at the American Society of Breast Surgeons’ Annual Meeting, 10-14 April 2024, Orlando FL.
Figures

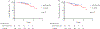
References
-
- Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer registry. Cancer. 2007;109(9):1721–8. 10.1002/cncr.22618. - DOI - PubMed
-
- Shepherd JH, Ballman K, Polley MC, et al. CALGB 40603 (Alliance): long-term outcomes and genomic correlates of response and survival after neoadjuvant chemotherapy with or without carboplatin and bevacizumab in triple-negative breast cancer. J Clin Oncol. 2022;40(12):1323–34. 10.1200/JCO.21.01506. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

