Complement activation by IgG subclasses is governed by their ability to oligomerize upon antigen binding
- PMID: 39436656
- PMCID: PMC11536094
- DOI: 10.1073/pnas.2406192121
Complement activation by IgG subclasses is governed by their ability to oligomerize upon antigen binding
Abstract
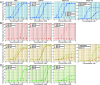
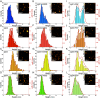
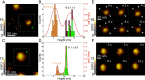
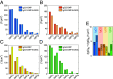
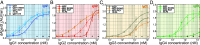
Complement activation through antibody-antigen complexes is crucial in various pathophysiological processes and utilized in immunotherapies to eliminate infectious agents, regulatory immune cells, or cancer cells. The tertiary structures of the four IgG antibody subclasses are largely comparable, with the most prominent difference being the hinge regions connecting the Fab and Fc domains, providing them with unique structural flexibility. Complement recruitment and activation depend strongly on IgG subclass, which is commonly rationalized by differences in hinge flexibility and the respective affinities for C1, the first component of the classical complement pathway. However, a unifying mechanism of how these different IgG subclass properties combine to modulate C1 activation has not yet been proposed. We here demonstrate that complement activation is determined by their varying ability to form IgG oligomers on antigenic surfaces large enough to multivalently bind and activate C1. We directly visualize the resulting IgG oligomer structures and characterize their distribution by means of high-speed atomic force microscopy, quantify their complement recruitment efficiency from quartz crystal microbalance experiments, and characterize their ability to activate complement on tumor cell lines as well as in vesicle-based complement lysis assays. We present a mechanistic model of the multivalent interactions that govern C1 binding to IgG oligomers and use it to extract kinetic rate constants from real-time interaction data from which we further calculate equilibrium dissociation constants. Together, we provide a comprehensive view on the parameters that govern complement activation by the different IgG subclasses, which may inform the design of future antibody therapies.
Keywords: C1; Fc–Fc interactions; IgG oligomerization; IgG subclasses; classical complement pathway.
Conflict of interest statement
Competing interests statement:E.G.F.B., A.F.L, and F.J.B. are employees at Genmab BV and have ownership interests (including stocks, warrants, patents, etc.). J.P. received Genmab funding.
Figures



References
-
- Ugurlar D., et al. , Structures of C1-IgG1 provide insights into how danger pattern recognition activates complement. Science 359, 794–797 (2018). - PubMed
-
- Schumaker V. N., Calcott M. A., Spiegelberg H. L., Mueller-Eberhard H. J., Ultracentrifuge studies of the binding of IgG of different subclasses to the Clq subunit of the first component of complement. Biochemistry 15, 5175–5181 (1976). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

