Targeting Unc5b in macrophages drives atherosclerosis regression and pro-resolving immune cell function
- PMID: 39436659
- PMCID: PMC11536151
- DOI: 10.1073/pnas.2412690121
Targeting Unc5b in macrophages drives atherosclerosis regression and pro-resolving immune cell function
Abstract
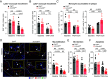
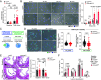
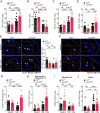
Atherosclerosis results from lipid-driven inflammation of the arterial wall that fails to resolve. Imbalances in macrophage accumulation and function, including diminished migratory capacity and defective efferocytosis, fuel maladaptive inflammation and plaque progression. The neuroimmune guidance cue netrin-1 has dichotomous roles in inflammation partly due to its multiple receptors; in atherosclerosis, netrin-1 promotes macrophage survival and retention via its receptor Unc5b. To minimize the pleiotropic effects of targeting netrin-1, we tested the therapeutic potential of deleting Unc5b in mice with advanced atherosclerosis. We generated Unc5bfl/flCx3cr1creERT2/WT mice, which allowed conditional deletion of Un5b (∆Unc5bMØ) in monocytes and macrophages by tamoxifen injection. After inducing advanced atherosclerosis by hepatic PCSK9 overexpression and western diet feeding for 20 wk, Unc5b was deleted and hypercholesterolemia was normalized to simulate clinical lipid management. Deletion of myeloid Unc5b led to a 40% decrease in atherosclerotic plaque burden and reduced plaque complexity compared to Unc5bfl/flCx3cr1WT/WT littermate controls (CtrlMØ). Consistently, plaque macrophage content was reduced by 50% in ∆Unc5bMØ mice due to reduced plaque Ly6Chi monocyte recruitment and macrophage retention. Compared to CtrlMØ mice, plaques in ∆Unc5bMØ mice had reduced necrotic area and fewer apoptotic cells, which correlated with improved efferocytotic capacity by Unc5b-deficient macrophages in vivo and in vitro. Beneficial changes in macrophage dynamics in the plaque upon Unc5b deletion were accompanied by an increase in atheroprotective T cell populations, including T-regulatory and Th2 cells. Our data identify Unc5b in advanced atherosclerosis as a therapeutic target to induce pro-resolving restructuring of the plaque immune cells and to promote atherosclerosis regression.
Keywords: Treg; atherosclerosis; efferocytosis; inflammation; regression.
Conflict of interest statement
Competing interests statement:K.J.M. is on the scientific advisory board of Beren Therapeutics and Bitterroot Bio. The other authors declare no conflict of interest. E.A.F. and K.J.M. have a patent on the use of inhibitors of Unc5b for treating inflammatory arthritis and KJM has a patent on the use of inhibitors of Unc5b for osteolysis.
Figures


References
-
- Tsao C. W., et al. , Heart disease and stroke statistics-2022 update: A report From the American Heart association. Circulation 145, e153–e639 (2022). - PubMed
-
- Sabatine M. S., et al. , Evolocumab and clinical outcomes in patients with cardiovascular disease. N. Engl. J. Med. 376, 1713–1722 (2017). - PubMed
-
- Schwartz G. G., et al. , Alirocumab and cardiovascular outcomes after acute coronary syndrome. N. Engl. J. Med. 379, 2097–2107 (2018). - PubMed
MeSH terms
Substances
Grants and funding
- P01 HL131481/HL/NHLBI NIH HHS/United States
- F30 HL167568/HL/NHLBI NIH HHS/United States
- 23SCEFIA1153739/American Heart Association (AHA)
- 19POST34380010/American Heart Association (AHA)
- 23POST1029885/American Heart Association (AHA)
- F30HL167568/HHS | NIH | NHLBI | Division of Intramural Research (DIR)
- R35 HL135799/HL/NHLBI NIH HHS/United States
- 915560/American Heart Association (AHA)
- MFE-176524/Canadian Government | Canadian Institutes of Health Research (CIHR)
- R35HL135799/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
- P01HL131481/HHS | NIH | National Heart, Lung, and Blood Institute (NHLBI)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

