Attenuated kidney oxidative metabolism in young adults with type 1 diabetes
- PMID: 39436695
- PMCID: PMC11645151
- DOI: 10.1172/JCI183984
Attenuated kidney oxidative metabolism in young adults with type 1 diabetes
Abstract
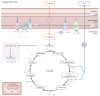
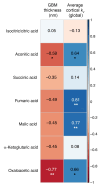
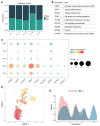
BACKGROUNDIn type 1 diabetes (T1D), impaired insulin sensitivity may contribute to the development of diabetic kidney disease (DKD) through alterations in kidney oxidative metabolism.METHODSYoung adults with T1D (n = 30) and healthy controls (HCs) (n = 20) underwent hyperinsulinemic-euglycemic clamp studies, MRI, 11C-acetate PET, kidney biopsies, single-cell RNA-Seq, and spatial metabolomics to assess this relationship.RESULTSParticipants with T1D had significantly higher glomerular basement membrane (GBM) thickness compared with HCs. T1D participants exhibited lower insulin sensitivity and cortical oxidative metabolism, correlating with higher insulin sensitivity. Proximal tubular transcripts of TCA cycle and oxidative phosphorylation enzymes were lower in T1D. Spatial metabolomics showed reductions in tubular TCA cycle intermediates, indicating mitochondrial dysfunction. The Slingshot algorithm identified a lineage of proximal tubular cells progressing from stable to adaptive/maladaptive subtypes, using pseudotime trajectory analysis, which computationally orders cells along a continuum of states. This analysis revealed distinct distribution patterns between T1D and HCs, with attenuated oxidative metabolism in T1D attributed to a greater proportion of adaptive/maladaptive subtypes with low expression of TCA cycle and oxidative phosphorylation transcripts. Pseudotime progression associated with higher HbA1c, BMI, and GBM, and lower insulin sensitivity and cortical oxidative metabolism.CONCLUSIONThese early structural and metabolic changes in T1D kidneys may precede clinical DKD.TRIAL REGISTRATIONClinicalTrials.gov NCT04074668.FUNDINGUniversity of Michigan O'Brien Kidney Translational Core Center grant (P30 DK081943); CROCODILE studies by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (P30 DK116073), Juvenile Diabetes Research Foundation (JDRF) (2-SRA-2019-845-S-B), Boettcher Foundation, Intramural Research Program at NIDDK and Centers for Disease Control and Prevention (CKD Initiative) under Inter-Agency Agreement #21FED2100157DPG.
Keywords: Diabetes; Endocrinology; Metabolism.
Figures






References
-
- Rapaport R, Sills IN. Implications of the DCCT for children and adolescents with IDDM. N J Med. 1994;91(4):227–228. - PubMed
-
- Purnell JQ, et al. Impact of excessive weight gain on cardiovascular outcomes in type 1 diabetes: results from the diabetes control and complications trial/epidemiology of diabetes interventions and complications (DCCT/EDIC) Study. Diabetes Care. 2017;40(12):1756–1762. doi: 10.2337/dc16-2523. - DOI - PMC - PubMed

