Increased expression of cathepsin C in airway epithelia exacerbates airway remodeling in asthma
- PMID: 39436705
- PMCID: PMC11601913
- DOI: 10.1172/jci.insight.181219
Increased expression of cathepsin C in airway epithelia exacerbates airway remodeling in asthma
Abstract
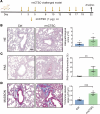
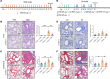
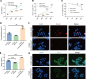
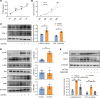
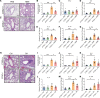
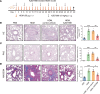
Airway remodeling is a critical factor determining the pathogenesis and treatment sensitivity of severe asthma (SA) or uncontrolled asthma (UA). The activation of epithelial-mesenchymal trophic units (EMTUs) regulated by airway epithelial cells (AECs) has been proven to induce airway remodeling directly. However, the triggers for EMTU activation and the underlying mechanism of airway remodeling are not fully elucidated. Here, we screened the differentially expressed gene cathepsin C (CTSC; also known as dipeptidyl peptidase 1 [DPP-1]) in epithelia of patients with SA and UA using RNA-sequencing data and further verified the increased expression of CTSC in induced sputum of patients with asthma, which was positively correlated with severity and airway remodeling. Moreover, direct instillation of exogenous CTSC induced airway remodeling. Genetic inhibition of CTSC suppressed EMTU activation and airway remodeling in two asthma models with airway remodeling. Mechanistically, increased secretion of CTSC from AECs induced EMTU activation through the p38-mediated pathway, further inducing airway remodeling. Meanwhile, inhibition of CTSC also reduced the infiltration of inflammatory cells and the production of inflammatory factors in the lungs of asthmatic mice. Consequently, targeting CTSC with compound AZD7986 protected against airway inflammation, EMTU activation, and remodeling in the asthma model. Based on the dual effects of CTSC on airway inflammation and remodeling, CTSC is a potential biomarker and therapeutic target for SA or UA.
Keywords: Asthma; Pulmonology; Respiration; Therapeutics.
Figures