A Programmable Handheld Extrusion-Based Bioprinting Platform for In Situ Skin Wounds Dressing: Balance Mobility and Customizability
- PMID: 39436787
- PMCID: PMC11633465
- DOI: 10.1002/advs.202405823
A Programmable Handheld Extrusion-Based Bioprinting Platform for In Situ Skin Wounds Dressing: Balance Mobility and Customizability
Abstract
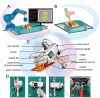
Bioprinting technology plays a crucial role for constructing tissue substitutes. However, the mismatched scaffold shapes and the poor treatment timeliness limit its clinical translational application. In situ printing technology that prints bioregenerants directly inside patient's body can meet the needs of specific tissue repair. This study develops a smartphone controlled handheld bioprinter for in situ skin wounds dressing. The mini bioprinter can be handheld and placed on any printing surface to create strips, complex patterns, and 3D structures, and can be equipped with microchannel needles to expand functionality. The size of the strips as well as the printing path can be programmed and controlled by the smartphone to ensure the precision of the printed product quality. Furthermore, the device not only allows for smooth switching between different bioinks for printing heterogeneous structure, but also allows for fast and uniform coverage of large wound surfaces. When dealing with complex wounds in vitro & vivo, the printer can effectively fill and precisely close wounds, promoting wound healing. The programmable handheld bioprinter can balance mobility and customizability in the management of skin wounds and is expected to realize its potential for emergency medical treatment in condition-constrained scenarios, such as battlefields or disaster areas.
Keywords: handheld printers; in situ bioprinting; programmable; skin regeneration.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- a) Fonseca A. C., Melchels F. P. W., Ferreira M. J. S., Moxon S. R., Potjewyd G., Dargaville T. R., Kimber S. J., Domingos M., Chem. Rev. 2020, 120, 11093; - PMC - PubMed
- b) Wang X., Luo Y., Ma Y., Wang P., Yao R., Trends Biotechnol. 2023, 42, 648; - PubMed
- c) Daly A. C., Prendergast M. E., Hughes A. J., Burdick J. A., Cell 2021, 184, 18. - PMC - PubMed
-
- a) O'Connor C., Brady E., Zheng Y., Moore E., Stevens K. R., Nat. Rev. Mater. 2022, 7, 702; - PMC - PubMed
- b) Wang Z. J., Liang X., Wang G. Y., Wang X. H., Chen Y., Adv. Mater. 2023, 2304738;
- c) Kang H. W., Lee S. J., Ko I. K., Kengla C., Yoo J. J., Atala A., Nat. Biotechnol. 2016, 34, 312; - PubMed
- d) de Melo B. A. G., Jodat Y. A., Mehrotra S., Calabrese M. A., Kamperman T., Mandal B. B., Santana M. H. A., Alsberg E., Leijten J., Shin S. R., Adv. Funct. Mater. 2019, 29, 1906330; - PMC - PubMed
- e) Wang C. M., Honiball J. R., Lin J. Y., Xia X. Y., Lau D. S. A., Chen B., Deng L. F., Lu W. W., ACS Appl. Mater. Interfaces 2022, 14, 27575. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
