Preclinical assessment of MAGMAS inhibitor as a potential therapy for pediatric medulloblastoma
- PMID: 39436961
- PMCID: PMC11495579
- DOI: 10.1371/journal.pone.0300411
Preclinical assessment of MAGMAS inhibitor as a potential therapy for pediatric medulloblastoma
Abstract
Medulloblastoma is the most common malignant brain tumor in children. It has WNT-driven, SHH-driven/TP53 mutant, SHH-driven/TP53 wildtype, and non-WNT/non-SHH subgroups. MAGMAS (Mitochondrial Associated Granulocyte Macrophage colony-stimulating factor Signaling molecules) encodes a mitochondrial import inner membrane translocase subunit and is responsible for the translocation of matrix proteins across the inner membrane. We previously reported that a small molecule MAGMAS inhibitor, BT9, decreases cell proliferation, migration, and oxidative phosphorylation in adult glioblastoma cell lines. The aim of our study was to investigate whether the chemotherapeutic effect of BT9 can be extended to pediatric medulloblastoma.
Methods: DAOY (SHH driven/tp53 mutant) and D425 (non-SHH group 3) were treated with BT9. For in vitro analysis, cell proliferation, death, migration, invasion, and metabolic activity were assessed using MTT assay, TUNEL staining, scratch wound assay, Matrigel invasion chambers, and seahorse assay, respectively. A D425 orthotopic xenograft mouse model was used to evaluate BT9 efficacy in vivo.
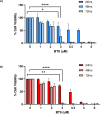
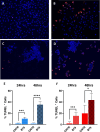
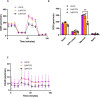
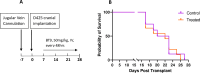
Results: BT9 treatment resulted in a significant decrease in cell proliferation (DAOY, 24 hours IC50: 3.6 μM, 48 hours IC50: 2.3 μM, 72 hours IC50: 2.1 μM; D425 24 hours IC50: 3.4 μM, 48 hours IC50: 2.2 μM, 72 hours IC50: 2.1 μM) and a significant increase in cell death (DAOY, 24 hours p = 0.0004, 48 hours p<0.0001; D425, 24 hours p = 0.0001, 48 hours p = 0.02). In DAOY cells, 3 μM BT9 delayed migration and significantly reduced DAOY and D425 cell invasion (p < 0.0001). It also modified mitochondrial respiratory function in both medulloblastoma cell lines. Compared to control, however, BT9 administration did not improve survival in a D425 orthotopic xenograft mouse model.
Conclusions: Our in vitro data showed BT9 antitumor efficacy in DAOY and D425 cell lines, suggesting that BT9 may represent a promising targeted therapeutic in pediatric medulloblastoma. These data, however, need to be further validated in animal models.
Copyright: © 2024 Motahari et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Update of
-
Preclinical assessment of MAGMAS inhibitor as a potential therapy for pediatric medulloblastoma.bioRxiv [Preprint]. 2024 Mar 3:2024.02.29.582709. doi: 10.1101/2024.02.29.582709. bioRxiv. 2024. Update in: PLoS One. 2024 Oct 22;19(10):e0300411. doi: 10.1371/journal.pone.0300411. PMID: 38464047 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

