Critical role of hepsin/TMPRSS1 in hearing and tectorial membrane morphogenesis: Insights from transgenic mouse models
- PMID: 39437584
- PMCID: PMC11531994
- DOI: 10.1016/j.heares.2024.109134
Critical role of hepsin/TMPRSS1 in hearing and tectorial membrane morphogenesis: Insights from transgenic mouse models
Abstract
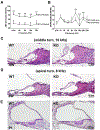
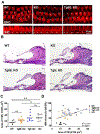
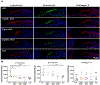
Mutations in various type II transmembrane serine protease (TMPRSS) family members are associated with non-syndromic hearing loss, with some mechanisms still unclear. For instance, the mechanism underlying profound hearing loss and tectorial membrane (TM) malformations in hepsin/TMPRSS1 knockout (KO) mice remains elusive. In this study, we confirmed significantly elevated hearing thresholds and abnormal TM morphology in hepsin KO mice, characterized by enlarged TM with gaps and detachment from the spiral limbus. Transgenic mouse lines were created to express either wild-type or a serine protease-dead mutant of human hepsin in the KO background. The Tg68;KO line, expressing moderate levels of wild-type human hepsin in the cochlea, showed partial restoration of hearing function. Conversely, the Tg5;KO or TgRS;KO lines, with undetectable hepsin or protease-dead hepsin, did not show such improvement. Histological analyses revealed that Tg68;KO mice, but not Tg5;KO or TgRS;KO mice, had a more compact TM structure, partially attached to the spiral limbus. These results indicate that hepsin expression levels correlate with improvements in hearing and TM morphology, and its protease activity is critical for these effects. Hepsin's role was further examined by studying its relationship with α-tectorin (TECTA) and β-tectorin (TECTB), non-collagenous proteins crucial for TM formation. Hepsin was co-expressed with TECTA and TECTB in the developing cochlear epithelium. Immunostaining showed decreased levels of TECTA and TECTB in hepsin KO TM, partially restored in Tg68;KO mice. These findings suggest that hepsin is essential for proper TM morphogenesis and auditory function, potentially by proteolytic processing/maturation of TECTA and TECTB and their incorporation into the TM.
Keywords: Hearing function; Hepsin; Tectorial membrane; Tectorin; Transgenic mice; Type II transmembrane serine protease.
Copyright © 2024 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest None.
Figures



References
-
- Brunati M, Perucca S, Han L, Cattaneo A, Consolato F, Andolfo A, Schaeffer C, Olinger E, Peng J, Santambrogio S, Perrier R, Li S, Bokhove M, Bachi A, Hummler E, Devuyst O, Wu Q, Jovine L, Rampoldi L, 2015. The serine protease hepsin mediates urinary secretion and polymerisation of Zona Pellucida domain protein uromodulin. Elife 4, e08887. 10.7554/eLife.08887. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

