Yeast-based assay to identify inhibitors of the malaria parasite sodium phosphate uptake transporter as potential novel antimalarial drugs
- PMID: 39437596
- PMCID: PMC11532756
- DOI: 10.1016/j.ijpddr.2024.100567
Yeast-based assay to identify inhibitors of the malaria parasite sodium phosphate uptake transporter as potential novel antimalarial drugs
Abstract
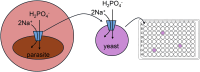
Malaria affects almost 250 million people annually and continues to be a significant threat to global public health. Infection with protozoan parasites from the genus Plasmodium causes malaria. The primary treatment for malaria is artemisinin-based combination therapies (ACTs). The spread of ACT-resistant parasites has undermined efforts to control and eradicate malaria. Thus, it is crucial to identify new targets for the development of novel antimalarial drugs. Phosphate is an essential nutrient for all cells. The Plasmodium falciparum genome encodes a single sodium-coupled inorganic phosphate transporter named PfPiT that is essential for parasite proliferation in the asexual blood stage. Thus, PfPiT inhibitors may be promising antimalarial drugs. Like Plasmodium, yeast requires phosphate to grow. We developed a Saccharomyces cerevisiae based growth assay to identify inhibitors of PfPiT. Genome editing was used to create a yeast strain where PfPiT was the only phosphate transporter. Using a radioactive [32P]phosphate uptake assay, the measured phosphate Km for PfPiT in yeast was 56 ± 7 μM in 1 mM NaCl at pH 7.4. The Km decreased to 24 ± 3 μM in 25 mM NaCl consistent with it being a Na+ coupled cotransporter. Conditions under which yeast growth was dependent on phosphate uptake mediated by PfPiT were identified and a 22-h growth assay was developed to screen for PfPiT inhibitors. In a screen of 21 compounds, two compounds were identified that inhibited the growth of the PfPiT strain but not that of the parental strain expressing Pho84, one of the five endogenous yeast phosphate transporters. Radioactive phosphate uptake experiments confirmed inhibition of phosphate uptake by the two compounds. The growth inhibition assay provides a simple and inexpensive approach to screen a large compound library for PfPiT inhibitors that may serve as starting points for the development of novel antimalarial drugs.
Keywords: Assay development; Drug discovery; Malaria; PfPiT; Plasmodium falciparum; Sodium phosphate cotransporters.
Copyright © 2024 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors report no conflicts of interest relevant to this manuscript.
Figures






References
-
- Auesukaree C., Homma T., Kaneko Y., Harashima S. Transcriptional regulation of phosphate-responsive genes in low-affinity phosphate-transporter-defective mutants in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 2003;306:843–850. - PubMed
-
- Balikagala B., Fukuda N., Ikeda M., Katuro O.T., Tachibana S.I., Yamauchi M., Opio W., Emoto S., Anywar D.A., Kimura E., Palacpac N.M.Q., Odongo-Aginya E.I., Ogwang M., Horii T., Mita T. Evidence of artemisinin-resistant malaria in Africa. N. Engl. J. Med. 2021;385:1163–1171. - PubMed
-
- Brunton L.L., Knollmann B.C. fourteenth ed., fourteenth ed. McGraw Hill Education; New York: 2023. Goodman & Gilman's the Pharmacological Basis of Therapeutics.
-
- Dallaire L., Béliveau R. Phosphate transport by capillaries of the blood-brain barrier. J. Biol. Chem. 1992;267:22323–22327. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

