Zanubrutinib, obinutuzumab, and venetoclax for first-line treatment of mantle cell lymphoma with a TP53 mutation
- PMID: 39437708
- PMCID: PMC11826521
- DOI: 10.1182/blood.2024025563
Zanubrutinib, obinutuzumab, and venetoclax for first-line treatment of mantle cell lymphoma with a TP53 mutation
Abstract
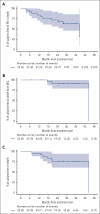
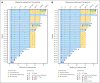
TP53-mutant mantle cell lymphoma (MCL) is associated with poor survival outcomes with standard chemoimmunotherapy. We conducted a multicenter, phase 2 study of zanubrutinib, obinutuzumab, and venetoclax (BOVen) in untreated patients with MCL with a TP53 mutation. Patients initially received 160 mg zanubrutinib twice daily and obinutuzumab. Obinutuzumab at a dose of 1000 mg was given on cycle 1 day 1, 8, and 15, and on day 1 of cycles 2 to 8. After 2 cycles, venetoclax was added with weekly dose ramp-up to 400 mg daily. After 24 cycles, if patients were in complete remission with undetectable minimal residual disease (uMRD) using an immunosequencing assay, treatment was discontinued. The primary end point was met if ≥11 patients were progression free at 2 years. The study included 25 patients with untreated MCL with a TP53 mutation. The best overall response rate was 96% (24/25) and the complete response rate was 88% (22/25). Frequency of uMRD at a sensitivity level of 1 × 10-5 and uMRD at a sensitivity level of 1 × 10-6 at cycle 13 was 95% (18/19) and 84% (16/19), respectively. With a median follow-up of 28.2 months, the 2-year progression-free, disease-specific, and overall survival were 72%, 91%, and 76%, respectively. Common side effects were generally low grade and included diarrhea (64%), neutropenia (32%), and infusion-related reactions (24%). BOVen was well tolerated and met its primary efficacy end point in TP53-mutant MCL. These data support its use and ongoing evaluation. This trial was registered at www.ClinicalTrials.gov as #NCT03824483.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: A.K. reports receiving research support from AbbVie, Adaptive Biotechnologies, Celgene, Pharmacyclics, Loxo/Eli Lilly Pharmaceuticals, Seattle Genetics, Genentech, and Incyte; serving as a consultant for Adaptive Biotechnologies, AstraZeneca, Kite Pharmaceuticals, Janssen, Genentech, and Loxo/Eli Lilly Pharmaceuticals; and serving in a consultation role for Genentech. A.D.Z. reports receiving research support from MEI Pharmaceuticals, Genentech/Roche, and BeiGene; serving as a consultant for Genentech/Roche, Gilead, Celgene, Janssen, Amgen, Novartis, Adaptive Biotechnologies, MorphoSys, AbbVie, AstraZeneca, and MEI Pharmaceuticals; and serving as the data monitoring committee chair for BeiGene and as a data monitoring committee member for Bristol Myers Squibb (BMS), Celgene, and Juno. J.S. reports receiving research support from Adaptive Biotechnologies, BeiGene, BostonGene, Genentech/Roche, GlaxoSmithKline, Moderna, Takeda, and TG Therapeutics and serving as a consultant for AstraZeneca, BMS, Genentech/Roche, and Loxo/Eli Lilly. L.F. reports receiving research support from Roche, Genentech, Genmab, AbbVie, Innate Pharma, and BeiGene; serving as a consultant for Roche, Genentech, Genmab, AbbVie, Sanofi, and EvolveImmune; serving on the advisory board for AbbVie, Genentech, ADC Therapeutics, Seagen, and Ipsen; and receiving travel support from Genmab and AbbVie. A.D. reports receiving research support from Roche and AstraZeneca. J.K.L. reports receiving research support from Kymera Therapeutics; and serving as a consultant for ADC Therapeutics, Merck, Genmab, and AbbVie. J.S.A. reports serving as a consultant for AbbVie, AstraZeneca, BeiGene, Genentech, Janssen, Lilly, and Roche. P.C.J. reports receiving research support from Medically Home, AstraZeneca, and Incyte; and serving as a consultant for AstraZeneca, ADC Therapeutics, AbbVie, Seagen, Incyte, and BMS. J.E.H. reports serving as a consultant for and receiving research funding from Genmab. The remaining authors declare no competing financial interests.
Figures


References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

