Elritercept, a modified activin receptor IIA ligand trap, increased erythropoiesis and thrombopoiesis in a phase 1 trial
- PMID: 39437803
- PMCID: PMC11758838
- DOI: 10.1182/bloodadvances.2024014172
Elritercept, a modified activin receptor IIA ligand trap, increased erythropoiesis and thrombopoiesis in a phase 1 trial
Abstract
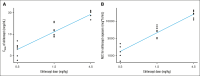
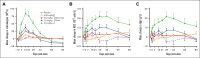
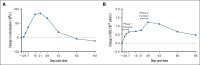
The transforming growth factor β (TGF-β) superfamily plays a crucial role in regulating biological processes of virtually every tissue and system in the body, including hemostasis and hematopoiesis. Elritercept (KER-050) is an investigational, modified activin receptor type IIA ligand trap designed to bind and inhibit activin A and other select TGF-β superfamily ligands, including activin B, growth differentiation factor 8 (GDF-8), and GDF-11. The objectives of this phase 1 randomized, placebo-controlled study of elritercept were to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamic markers of activin inhibition and hematopoiesis in healthy postmenopausal women (N = 48). This study comprised 2 parts: single ascending doses ranging from 0.05 to 4.5 mg/kg; and multiple (up to 2 doses) ascending doses of 0.75 mg/kg administered subcutaneously (SC) every 4 weeks. Elritercept was generally well tolerated at all dose levels, with no dose-limiting toxicities observed. There were no severe or serious adverse events or clinically significant changes in safety laboratory measures. Serum concentrations increased in a dose-proportional manner after single SC doses, with peak concentrations achieved in 4.5 to 6 days and a mean elimination half-life of 12 days. These parameters were comparable after multiple doses. Elritercept elicited rapid, sustained, and dose-dependent increases in reticulocytes, red blood cells, hemoglobin, and platelets without eliciting detrimental changes in white blood cells such as neutrophils and lymphocytes. The time course and duration of changes in these cell populations supported a differentiated pharmacologic profile that is consistent with the stimulation of both early- and late-stage hematologic pathways. The trial was registered at www.anzctr.org.au/ as #ACTRN12619000318189.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: J.L., C.R., S.B., J.T., H.N., and J.S. are current employees of or consultants for the study sponsor, Keros Therapeutics. B.S. declares no competing financial interests.
Figures

References
-
- Blank U, Karlsson S. TGF-β signaling in the control of hematopoietic stem cells. Blood. 2015;125(23):3542–3550. - PubMed
-
- Platzbecker U, Germing U, Gotze KS, et al. Luspatercept for the treatment of anaemia in patients with lower-risk myelodysplastic syndromes (PACE-MDS): a multicentre, open-label phase 2 dose-finding study with long-term extension study. Lancet Oncol. 2017;18(10):1338–1347. - PubMed
-
- Fenaux P, Platzbecker U, Mufti GJ, et al. Luspatercept in patients with lower-risk myelodysplastic syndromes. N Engl J Med. 2020;382(2):140–151. - PubMed
-
- Ruckle J, Jacobs M, Kramer W, et al. Single-dose, randomized, double-blind, placebo-controlled study of ACE-011 (ActRIIA-IgG1) in postmenopausal women. J Bone Miner Res. 2009;24(4):744–752. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

