Pathological response following neoadjuvant immune checkpoint inhibitors in patients with hepatocellular carcinoma: a cross-trial, patient-level analysis
- PMID: 39437804
- PMCID: PMC12040480
- DOI: 10.1016/S1470-2045(24)00457-1
Pathological response following neoadjuvant immune checkpoint inhibitors in patients with hepatocellular carcinoma: a cross-trial, patient-level analysis
Abstract
Background: Neoadjuvant use of immune checkpoint inhibitors (ICIs) before liver resection results in pathological tumour regression in patients with hepatocellular carcinoma. We aimed to describe the characteristics of pathological responses after preoperative ICI therapy for hepatocellular carcinoma and to evaluate the association between the depth of tumour regression and relapse-free survival.
Methods: In this cross-trial, patient-level analysis, we performed a pooled analysis of data from patients with hepatocellular carcinoma receiving ICI therapy before liver resection as part of a global collaborative consortium (NeoHCC) of five phase 1 and 2 clinical trials and standardised observational protocols conducted in 12 tertiary referral centres across the USA, UK, and Taiwan. Eligible patients were adults (aged ≥18 years) diagnosed with hepatocellular carcinoma by tissue core biopsy before treatment initiation, a Liver Imaging Reporting and Data System score of 5 on imaging, or both, with an Eastern Cooperative Oncology Group performance status score of 0-1, and no extrahepatic spread or previous ICI treatment. Pathological response was measured as the percentage of non-viable tumour in the resected surgical specimen, with major pathological response corresponding to at least 70% tumour regression and pathological complete response corresponding to 100% tumour regression. We correlated pathological response with radiological overall response using RECIST criteria (version 1.1) and relapse-free survival, and evaluated the threshold of tumour regression that could be optimally associated with relapse-free survival.
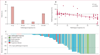
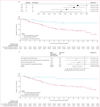
Findings: At data cutoff on Jan 31, 2024, 111 patients were included in the study, of whom data on pathological response were available for 104 (94%) patients. Patients received treatment from Oct 5, 2017, to Nov 15, 2023, mostly ICI combinations (76 [69%]), for a median of 1·4 months (IQR 0·7-2·9). 87 (78%) patients were men and 24 (22%) were women. Most patients had underlying viral chronic liver disease (73 [66%]) and Barcelona Clinic Liver Cancer stage A hepatocellular carcinoma (61 [55%]), without portal vein thrombosis (87 [78%]). We observed major pathological response in 33 (32%) patients and pathological complete response in 19 (18%) patients. Radiological overall response was associated with major pathological response, with 23 (74%) of 31 patients with radiological response showing major pathological response compared with ten (14%) of 73 patients without radiological response (p<0·0001). However, ten (30%) of 33 major pathological responses were not predicted by radiological response. After a median follow-up of 27·2 months (95% CI 22·3-32·1), median relapse-free survival for the whole cohort was 43·6 months (95% CI 28·3-not evaluable). Relapse-free survival was significantly longer in patients with major pathological response than in those who did not have a major pathological response (not reached [95% CI not evaluable-not evaluable] vs 28·3 months [12·8-43·8]; hazard ratio 0·26 [0·10-0·66]; p=0·0024) and in patients with pathological complete response than in those who did not have a pathological complete response (NR [95% CI not evaluable-not evaluable] vs 32·8 months [15·0-50·5]; 0·19 [0·05-0·78]; p=0·010). Unbiased recursive partitioning of the cohort for the risk of relapse, death, or both identified a threshold of 90% as the optimal cutoff of pathological tumour regression to predict improved relapse-free survival.
Interpretation: The extent of tumour regression following neoadjuvant ICI therapy could identify patients with improved relapse-free survival following liver resection. The threshold of at least 90% tumour regression should be validated for its surrogate role for relapse-free survival in phase 3 randomised controlled trials.
Funding: None.
Copyright © 2024 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license.
Conflict of interest statement
Declaration of interests AD received educational support for congress attendance and consultancy fees from Roche; and speaker fees from Roche, AstraZeneca, Eisai, and Chugai. SS reports consultancy fees from Eisai and Pfizer. CAMF reports speaker fees from EISAI. CC received consulting fees from Eisai and MSD; speaker fees from Eisai, MSD, AstraZeneca, and Ipsen; and travel expenses from Roche. SCW received speaker fees from AstraZeneca and Intercept; and research funding (to their institution) from Boehringer Ingelheim. AC received consulting fees from Regeneron, MSD, Bristol Myers Squibb, AstraZeneca, and Roche; speaker fees from Sanofi–Regeneron, MSD, Roche, and AstraZeneca; and travel expenses from MSD, Roche, and Sanofi–Regeneron. CH received speaker fees from AstraZeneca, Bristol Myers Squibb–ONO, and Roche; and research funding (to their institution) from National Science and Technology Council (Taiwan) and Bristol Myers Squibb–ONO. MB received consulting fees from AstraZeneca and Eisai. MY received speaker fees from Genentech, Exelixis, AstraZeneca, Replimune, Hepion, and Lantheus; grant or research support (to their institution) from the National Cancer Institute–National Institutes of Health, Fibrolamellar Cancer Foundation, Cancer Research Institute, Bristol Myers Squibb, Exelixis, Incyte, and Genentech; and is cofounder with equity of Adventris Pharmaceuticals. TUM received consulting fees from Avammune Therapeutics and ONO; participated on boards for Rockefeller, AbbVie, Celldex, Regeneron, AbbVie, Merck, Bristol-Meyers Squibb, Boehringer Ingelheim, Atara, AstraZeneca, Genentech, Chimeric, Glenmark, Simcere, Surface, G1 Therapeutics, NGM Bio, DBV Technologies, Arcus, Fate, EMD Serono, and Astellas; and received research funding (to their institution) from Regeneron, Genentech, Bristol Myers Squibb, Merck, and Boehringer Ingelheim. DJP received lecture fees from Roche, Bristol Myers Squibb, Eisai, and Boston Scientific; travel expenses from Roche, Bristol Myers Squibb, and MSD; consulting fees for Mina Therapeutics, Eisai, Roche, Avamune, Da Volterra, Mursla, H3B, Ipsen, Boston Scientific, Starpharma, Exact Sciences, and AstraZeneca; participated on advisory boards for Mina Therapeutics, Eisai, Roche, Avamune, Da Volterra, Mursla, H3B, Ipsen, LIfT Biosciences, Exact Sciences, and AstraZeneca; and received research funding (to their institution) from MSD, GSK, and Bristol Myers Squibb. All other authors declare no competing interests.
Figures

Comment in
-
Neoadjuvant immunotherapy for hepatocellular carcinoma: progress and perspectives.Hepatobiliary Surg Nutr. 2025 Feb 1;14(1):140-142. doi: 10.21037/hbsn-2024-642. Epub 2024 Dec 31. Hepatobiliary Surg Nutr. 2025. PMID: 39925908 Free PMC article. No abstract available.
References
-
- Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet 2022; 400: 1345–62. - PubMed
-
- Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg 2015; 261: 947–55. - PubMed
-
- Wang K, Xiang Y-J, Yu H-M, et al. Adjuvant sintilimab in resected high-risk hepatocellular carcinoma: a randomized, controlled, phase 2 trial. Nat Med 2024; 30: 708–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

