Glucagon-like peptide-1 receptor agonists before upper gastrointestinal endoscopy and risk of pulmonary aspiration or discontinuation of procedure: cohort study
- PMID: 39438043
- PMCID: PMC11494456
- DOI: 10.1136/bmj-2024-080340
Glucagon-like peptide-1 receptor agonists before upper gastrointestinal endoscopy and risk of pulmonary aspiration or discontinuation of procedure: cohort study
Abstract
Objective: To assess whether use of glucagon-like peptide-1 (GLP-1) receptor agonists before upper gastrointestinal endoscopy is associated with increased risk of pulmonary aspiration or discontinuation of the procedure compared with sodium-glucose cotransporter-2 (SGLT-2) inhibitors.
Design: Cohort study.
Setting: Two deidentified US commercial healthcare databases.
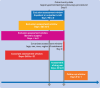
Participants: 43 365 adults (≥18 years) with type 2 diabetes who used a GLP-1 receptor agonist or SGLT-2 inhibitor within 30 days before upper gastrointestinal endoscopy.
Main outcome measures: The primary outcome was pulmonary aspiration on the day of or the day after endoscopy, defined using diagnostic codes. The secondary outcome was discontinuation of endoscopy. Risk ratios and corresponding 95% confidence intervals (CIs) were estimated after fine stratification weighting based on propensity score.
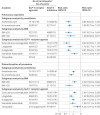
Results: After weighting, 24 817 adults used a GLP-1 receptor agonist (mean age 59.9 years; 63.6% female) and 18 537 used an SGLT-2 inhibitor (59.8 years; 63.7% female). Among users of GLP-1 receptor agonists and SGLT-2 inhibitors, the weighted risk per 1000 people was, respectively, 4.15 and 4.26 for pulmonary aspiration and 9.79 and 4.91 for discontinuation of endoscopy. Compared with SGLT-2 inhibitor use, GLP-1 receptor agonist use was not associated with an increased risk of pulmonary aspiration (pooled risk ratio 0.98, 95% CI 0.73 to 1.31), although it was associated with a higher risk for discontinuation of endoscopy (1.99, 1.56 to 2.53).
Conclusions: In this comparative cohort study, no increased risk of pulmonary aspiration during upper gastrointestinal endoscopy was observed among adults with type 2 diabetes using GLP-1 receptor agonists compared with SGLT-2 inhibitors within 30 days of the procedure; however, GLP-1 receptor agonists were associated with a higher risk of discontinuation of endoscopy, possibly owing to a higher risk of retained gastric content. In the absence of evidence from randomized trials, these findings could inform future practice recommendations on the preprocedural protocol for patients requiring endoscopy.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: SJC has a close family member employed by a Johnson & Johnson company and reports consultancy for Wolters-Kluwer and Alexion Pharmaceuticals. DJW reports consultancy for Novo Nordisk and serving as a data monitoring committee member for SOUL and FLOW trials. EP is principal investigator of a research grant to the Brigham and Women’s Hospital from Boehringer Ingelheim, not related to the topic of this work. CCT reports the following activities: Apollo Endosurgery – consultant/research support (consulting fees/institutional research grants); Bariendo – Founder/Board Member/Ownership Interest; BlueFlame Healthcare Venture Fund – Founder/General Partner; Boston Scientific – consultant (consulting fees)/research support (institutional research grant); Medtronic – consultant (consulting fees); ELLES – founder/board member/ownership interest; Endoquest Robotics – consultant, institutional research grant; Enterasense – founder, consultant, board member, ownership interest; EnVision Endoscopy – founder, board member, consultant, ownership interest; ERBE – institutional research grant; Fractyl – consultant/advisory board member (consulting fees)/research support; FujiFilm – consultant/institutional research grant; GI Dynamics – consultant (consulting fees)/ research support (institutional research grant); GI Windows – founder, board member, ownership interest; Lumendi – consultant/institutional research grant; Olympus/Spiration – consultant (consulting fees)/research support (Equipment Loans); Society for Metabolic and Bariatric Endoscopy (SMBE) – founder/president/ownership interest; Softac – consultant/ownership interest; USGI Medical – consultant (consulting fees)/advisory board member (consulting fees)/research support (institutional research grant); Xenter – consultant/SAB/ownership interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
