Combined programmed cell death protein 1 and cytotoxic T-lymphocyte associated protein 4 blockade in an international cohort of patients with acral lentiginous melanoma
- PMID: 39438074
- PMCID: PMC11758508
- DOI: 10.1093/bjd/ljae401
Combined programmed cell death protein 1 and cytotoxic T-lymphocyte associated protein 4 blockade in an international cohort of patients with acral lentiginous melanoma
Abstract
Background: Combination immune checkpoint blockade targeting programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte associated protein 4 (CTLA-4) leads to high response rates and improved survival in patients with advanced cutaneous melanoma (CM). Less is known about the efficacy of this combination in acral lentiginous melanoma (ALM).
Objectives: To determine the efficacy of combination immune checkpoint blockade targeting PD-1 and CTLA-4 in a diverse, real-world population of patients with ALM.
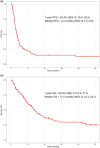
Methods: This multi-institutional retrospective study analysed patients with histologically confirmed ALM treated with a combination of PD-1 and CTLA-4 inhibitors between 2010 and 2022. The primary objective of the study was the objective response rate (ORR) as per the RECIST criteria. The secondary objectives were progression-free survival (PFS) and overall survival (OS).
Results: In total, 109 patients with advanced ALM treated with combined PD-1 and CTLA-4 blockade in any line of treatment were included. The majority of patients had stage IV disease (n = 81; 74.3%). The ORR for the entire cohort was 18.3% [95% confidence interval (CI) 11.6-26.9], with 9 (8.3%) complete and 11 (10.1%) partial responses. A further 22 patients (20.2%) had stable disease, and the disease control rate was 38.5%. Median PFS was 4.2 months (95% CI 3.25-5.62), while median OS was 17 months (95% CI 12.4-23.1). Ninety-five patients (87.2%) had a treatment-related adverse event, with 40.4% (n = 44/109) experiencing at least one grade 3 or 4 toxicity. Elevated lactate dehydrogenase (P = 0.04), ≥ 2 lines of prior treatment (P = 0.03) and Asian ethnicity (P = 0.04) were associated with worse OS, while Hispanic/Latino ethnicity was associated with better OS (P = 0.02).
Conclusions: Combination PD-1 and CTLA-4 blockade is less effective for ALM than for CM, despite similar toxicity. In particular, Asian patients appear to derive less benefit from this regimen. Novel treatment approaches are needed for this rare melanoma subtype.
Plain language summary
Melanoma is an aggressive type of skin cancer that affects more than 325,000 people worldwide every year. Acral lentiginous melanoma (or ‘ALM’ for short) is rare type of melanoma that appears on the palms of the hands, soles of the feet or in nailbeds. Unusually, it is not thought to be caused by exposure to the sun, unlike most other skin melanomas. Medications that use the body’s own immune system to fight cancer are very effective against advanced melanoma that is no longer suitable for surgery. This is called ‘immunotherapy’. Two thirds of people with advanced skin melanomas had their tumours shrink when they were treated with two immunotherapy medications used together. Immunotherapy appears to be less effective in ALM, but research has been very limited. In our international study that included more than 100 patients from multiple cancer centres, we looked at how effective two immunotherapy drugs were when used together against ALM in people whose cancer was advanced. We found that ALM is not as responsive to these two drug combinations when compared with other skin melanomas. Only about 2 in 10 patients with ALM in the study had their tumours decrease in size. We also found that combination immunotherapy had limited benefit on patients’ survival. Most cancers came back after about 4 months and led to patients dying after about 17 months. Our results suggest that combination immunotherapy is not very effective in ALM. New treatment options must be explored.
© The Author(s) 2024. Published by Oxford University Press on behalf of British Association of Dermatologists. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
Conflicts of interest: Unrelated to this work, C.M.C. has worked as the Principal Investigator with DeepX Health on melanoma diagnostic imaging technology. The funding was paid to his institution. G.K.I. is on advisory boards for BMS, Merck, Regeneron, Array, Castle Biosciences, Sanofi, Replimune and Pfizer. Institutional research support funds were provided by Regeneron, Array, Idera, Replimune, Xencor, InstilBio, Pfizer and Checkmate Pharmaceuticals. K.B.K. has received honoraria for serving as a speaker and member of an advisory board for Bristol Myers Squibb. He is also an Editorial Board Member/Editor-in-Chief/Associate Editor/Guest Editor for Melanoma Management and was not involved in the editorial review or the decision to publish this article. A.R.M. has received grants/research fundings from Kyowa, Miragen, Regeneron, Corbus, Sun Pharma, Incyte, Pfizer Inc, Merck & Co., Priovant Therapeutics, Eli Lilly, Elorac, Novartis, Janssen, Soligenix, Argenx, Palvella and AbbVie; and has served as a consultant for Kyowa, Eli Lilly, Momenta, UCB, Regeneron, Incyte, PHELEC, Soligenix, Clarivate, Argenx, Janssen, Bristol Myers Squibb, Boehringer Ingelheim and Pfizer. He has two provisional intellectual property (IP)/patents and one filed IP/patent (‘Methods and materials for assessing and treating cutaneous squamous cell carcinoma’ provisional 63-423254; ‘Use of oral Jaki in lichen planus’ provisional 63/453,065; and ‘Topical ruxolitinib in lichen planus’ wo2022072814a1, respectively). Y.G.N. is on the consulting/advisory board for Merck, BMS, Pfizer, Immunocore, Mallinkrodt, InterVenn Bio and Novartis. She is a speaker for Immunocore and Pfizer. The other authors declare no conflicts of interest.
Figures



Comment in
-
Real-world data confirms limited efficacy of dual programmed cell death protein 1 and cytotoxic T-lymphocyte associated protein 4 immune checkpoint blockade in acral lentiginous melanoma.Br J Dermatol. 2025 Jan 24;192(2):186-187. doi: 10.1093/bjd/ljae445. Br J Dermatol. 2025. PMID: 39545493 No abstract available.
References
-
- Elder DE, Bastian BC, Cree IA et al. The 2018 World Health Organization classification of cutaneous, mucosal, and uveal melanoma: detailed analysis of 9 distinct subtypes defined by their evolutionary pathway. Arch Pathol Lab Med 2020; 144:500–22. - PubMed
-
- Hayward NK, Wilmott JS, Waddell N et al. Whole-genome landscapes of major melanoma subtypes. Nature 2017; 545:175–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

