Oral reovirus reshapes the gut microbiome and enhances antitumor immunity in colon cancer
- PMID: 39438458
- PMCID: PMC11496807
- DOI: 10.1038/s41467-024-53347-6
Oral reovirus reshapes the gut microbiome and enhances antitumor immunity in colon cancer
Abstract
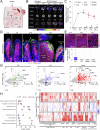
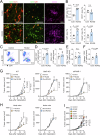
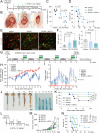
The route of oncolytic virotherapy is pivotal for immunotherapeutic efficacy in advanced cancers. In this preclinical study, an oncolytic reovirus (RC402) is orally administered to induce antitumor immunity. Oral reovirus treatment shows no gross toxicities and effectively suppresses multifocal tumor lesions. Orally administered reovirus interacts with the host immune system in the Peyer's patch of the terminal ileum, increases IgA+ antibody-secreting cells in the lamina propria through MAdCAM-1+ blood vessels, and reshapes the gut microbiome. Oral reovirus promotes antigen presentation, type I/II interferons, and T cell activation within distant tumors, but does not reach or directly infect tumor cells beyond the gastrointestinal tract. In contrast to intratumoral reovirus injection, the presence of the gut microbiome, Batf3+ dendritic cells, type I interferons, and CD8+ T cells are indispensable for orally administered reovirus-induced antitumor immunity. Oral reovirus treatment is most effective when combined with αPD-1(L1) and/or αCTLA-4, leading to complete colon tumor regression and protective immune memory. Collectively, oral reovirus virotherapy is a feasible and effective immunotherapeutic strategy in preclinical studies.
© 2024. The Author(s).
Conflict of interest statement
C.K. has a consulting or advisory role at Roche, MSD, BMS, ONO, Oncocross, Virocure, and Sillajen, and has received research grants from Panolos Bioscience, Boryung Pharmaceuticals, Oncocross, Sillajen, and Virocure. H.J.C. has a consulting or advisory role at Roche, Bayer, ONO, MSD, BMS, Celgene, Sanofi, Servier, AstraZeneca, Eisai, Sillajen, Menarini, GreenCross Cell, and Virocure and has received research grants from Panolos Bioscience, Roche and Boryung Pharmaceuticals. E.G., K-H.S. and D.G.P. are employees of Virocure. T.A. has a consulting and advisory role at Virocure, CuroV Science and Genvira Biosciences. The other authors declare no competing interests.
Figures



References
-
- Shalhout, S. Z., Miller, D. M., Emerick, K. S. & Kaufman, H. L. Therapy with oncolytic viruses: progress and challenges. Nat. Rev. Clin. Oncol.20, 160–177 (2023). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

