In situ visualization of endothelial cell-derived extracellular vesicle formation in steady state and malignant conditions
- PMID: 39438460
- PMCID: PMC11496675
- DOI: 10.1038/s41467-024-52867-5
In situ visualization of endothelial cell-derived extracellular vesicle formation in steady state and malignant conditions
Erratum in
-
Author Correction: In situ visualization of endothelial cell-derived extracellular vesicle formation in steady state and malignant conditions.Nat Commun. 2025 Oct 3;16(1):8813. doi: 10.1038/s41467-025-64770-8. Nat Commun. 2025. PMID: 41044152 Free PMC article. No abstract available.
Abstract
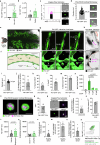
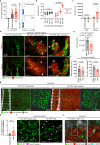
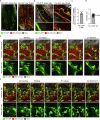
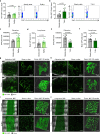
Endothelial cells are integral components of all vasculature within complex organisms. As they line the blood vessel wall, endothelial cells are constantly exposed to a variety of molecular factors and shear force that can induce cellular damage and stress. However, how endothelial cells are removed or eliminate unwanted cellular contents, remains unclear. The generation of large extracellular vesicles (EVs) has emerged as a key mechanism for the removal of cellular waste from cells that are dying or stressed. Here, we used intravital microscopy of the bone marrow to directly measure the kinetics of EV formation from endothelial cells in vivo under homoeostatic and malignant conditions. These large EVs are mitochondria-rich, expose the 'eat me' signal phosphatidylserine, and can interact with immune cell populations as a potential clearance mechanism. Elevated levels of circulating EVs correlates with degradation of the bone marrow vasculature caused by acute myeloid leukaemia. Together, our study provides in vivo spatio-temporal characterization of EV formation in the murine vasculature and suggests that circulating, large endothelial cell-derived EVs can provide a snapshot of vascular damage at distal sites.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Giannotta, M., Trani, M. & Dejana, E. VE-cadherin and endothelial adherens junctions: Aactive guardians of vascular integrity. Dev. Cell.26, 441–454 (2013). - PubMed
-
- Liebner, S., Cavallaro, U. & Dejana, E. The multiple languages of endothelial cell-to-cell communication. Arterioscler. Thromb. Vasc. Biol.26, 1431–1438 (2006). - PubMed
-
- Grellier, M. et al. Role of vascular endothelial growth factor in the communication between human osteoprogenitors and endothelial cells. J. Cell. Biochem.106, 390–398 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2008652/Department of Health | National Health and Medical Research Council (NHMRC)
- 2009287/Department of Health | National Health and Medical Research Council (NHMRC)
- 1140187/Department of Health | National Health and Medical Research Council (NHMRC)
- 9354/Cass Foundation
- 4852/Jack Brockhoff Foundation
LinkOut - more resources
Full Text Sources
Medical
Research Materials

