Advanced three-dimensional X-ray imaging unravels structural development of the human thymus compartments
- PMID: 39438572
- PMCID: PMC11496816
- DOI: 10.1038/s43856-024-00623-7
Advanced three-dimensional X-ray imaging unravels structural development of the human thymus compartments
Abstract
Background: The thymus, responsible for T cell-mediated adaptive immune system, has a structural and functional complexity that is not yet fully understood. Until now, thymic anatomy has been studied using histological thin sections or confocal microscopy 3D reconstruction, necessarily for limited volumes.
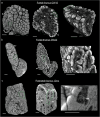
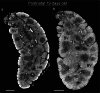
Methods: We used Phase Contrast X-Ray Computed Tomography to address the lack of whole-organ volumetric information on the microarchitecture of its structural components. We scanned 15 human thymi (9 foetal and 6 postnatal) with synchrotron radiation, and repeated scans using a conventional laboratory x-ray system. We used histology, immunofluorescence and flow cytometry to validate the x-ray findings.
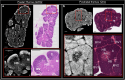
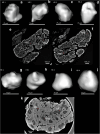
Results: Application to human thymi at pre- and post-natal stages allowed reliable tracking and quantification of the evolution of parameters such as size and distribution of Hassall's Bodies and medulla-to-cortex ratio, whose changes reflect adaptation of thymic activity. We show that Hassall's bodies can occupy 25% of the medulla volume, indicating they should be considered a third thymic compartment with possible implications on their role. Moreover, we demonstrate compatible results can be obtained with standard laboratory-based x-ray equipment, making this research tool accessible to a wider community.
Conclusions: Our study allows overcoming the resolution and/or volumetric limitations of existing approaches for the study of thymic disfunction in congenital and acquired disorders affecting the adaptive immune system.
Plain language summary
The thymus is the organ responsible for programming the immune system. It consists of two main compartments, named medulla and cortex. The medulla contains onion-shaped parts known as “Hassall’s bodies”. By imaging thymi at different stages of development with advanced x-ray methods, we gain understanding of changes that occur over time in 3D. We quantified how much of the thymus was occupied by these different components as they change with age, showing that Hassall’s bodies can take up 25% of the medulla, and should therefore be considered a proper part of the thymus with a purpose. Having a better understanding of the thymus can prove important in targeting conditions such as Down syndrome and thymic tumours, as well as provide information about structure.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Mizuki Nishino, S. K. A., Kocher, O. N., Robert, L., Thurer, P. M. B. & Hatabu, H. The Thymus: A Comprehensive Review. Radiogr2006, 335–348 (2006). - PubMed
-
- Gayathri, J., Bharathadevi, M. & Sivakami, T. a Study on Histogenesis of Thymus in Human Foetuses. Int. J. Anat. Res.7, 6811–6817 (2019).
-
- Varga, I., Pospisilova, V., Jablonska-Mestanova, V., Galfiova, P. & Polak, S. The thymus: picture review of human thymus (renatal development). Bratisl. Lek. Listy112, 368–376 (2011). - PubMed
Grants and funding
- 10005465/Innovate UK
- CiET1819/2/78/Royal Academy of Engineering
- 639429/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- CC0102/WT_/Wellcome Trust/United Kingdom
- EP/T005408/1/RCUK | Engineering and Physical Sciences Research Council (EPSRC)
LinkOut - more resources
Full Text Sources

