Exosomal miR-1a-3p derived from glucocorticoid-stimulated M1 macrophages promotes the adipogenic differentiation of BMSCs in glucocorticoid-associated osteonecrosis of the femoral head by targeting Cebpz
- PMID: 39438865
- PMCID: PMC11494760
- DOI: 10.1186/s12951-024-02923-5
Exosomal miR-1a-3p derived from glucocorticoid-stimulated M1 macrophages promotes the adipogenic differentiation of BMSCs in glucocorticoid-associated osteonecrosis of the femoral head by targeting Cebpz
Erratum in
-
Correction: Exosomal miR-1a-3p derived from glucocorticoid-stimulated M1 macrophages promotes the adipogenic differentiation of BMSCs in glucocorticoid-associated osteonecrosis of the femoral head by targeting Cebpz.J Nanobiotechnology. 2024 Nov 11;22(1):694. doi: 10.1186/s12951-024-02958-8. J Nanobiotechnology. 2024. PMID: 39523298 Free PMC article. No abstract available.
Abstract
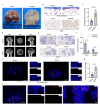
Background: By interacting with bone marrow mesenchymal stem cells (BMSCs) and regulating their function through exosomes, bone macrophages play crucial roles in various bone-related diseases. Research has highlighted a notable increase in the number of M1 macrophages in glucocorticoid-associated osteonecrosis of the femoral head (GA-ONFH). Nevertheless, the intricate crosstalk between M1 macrophages and BMSCs in the glucocorticoid-stimulated environment has not been fully elucidated, and the underlying regulatory mechanisms involved in the occurrence of GA-ONFH remain unclear.
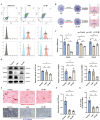
Methods: We employed in vivo mouse models and clinical samples from GA-ONFH patients to investigate the interactions between M1 macrophages and BMSCs. Immunofluorescence staining was used to assess the colocalization of M1 macrophages and BMSCs. Flow cytometry and transcriptomic analysis were performed to evaluate the impact of exosomes derived from normal (n-M1) and glucocorticoid-stimulated M1 macrophages (GC-M1) on BMSC differentiation. Additionally, miR-1a-3p expression was altered in vitro and in vivo to assess its role in regulating adipogenic differentiation.
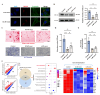
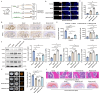
Results: In vivo, the colocalization of M1 macrophages and BMSCs was observed, and an increase in M1 macrophage numbers and a decrease in bone repair capabilities were further confirmed in both GA-ONFH patients and mouse models. Both n-M1 and GC-M1 were identified as contributors to the inhibition of osteogenic differentiation in BMSCs to a certain extent via exosome secretion. More importantly, exosomes derived from GC-M1 macrophages exhibited a heightened capacity to regulate the adipogenic differentiation of BMSCs, which was mediated by miR-1a-3p. In vivo and in vitro, miR-1a-3p promoted the adipogenic differentiation of BMSCs by targeting Cebpz and played an important role in the onset and progression of GA-ONFH.
Conclusion: We demonstrated that exosomes derived from GC-M1 macrophages disrupt the balance between osteogenic and adipogenic differentiation in BMSCs, contributing to the pathogenesis of GA-ONFH. Inhibiting miR-1a-3p expression, both in vitro and in vivo, significantly mitigates the preferential adipogenic differentiation of BMSCs, thus slowing the progression of GA-ONFH. These findings provide new insights into the regulatory mechanisms underlying GA-ONFH and highlight potential therapeutic targets for intervention.
Keywords: Adipogenic differentiation; Exosomes; GA-ONFH; miR-1a-3p.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Chang C, Greenspan A, Gershwin ME. The pathogenesis, diagnosis and clinical manifestations of steroid-induced osteonecrosis. J Autoimmun. 2020;110:102460. - PubMed
-
- Wang A, Ren M, Wang J. The pathogenesis of steroid-induced osteonecrosis of the femoral head: a systematic review of the literature. Gene. 2018;671:103–9. - PubMed
-
- Mont MA, Cherian JJ, Sierra RJ, Jones LC, Lieberman JR. Nontraumatic osteonecrosis of the femoral head: where do we stand today? A ten-year update. J Bone Joint Surg Am. 2015;97:1604–27. - PubMed
-
- Houdek MT, Wyles CC, Packard BD, Terzic A, Behfar A, Sierra RJ. Decreased osteogenic activity of mesenchymal stem cells in patients with corticosteroid-Induced osteonecrosis of the femoral head. J Arthroplasty. 2016;31:893–8. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

