Combining ERAP1 silencing and entinostat therapy to overcome resistance to cancer immunotherapy in neuroblastoma
- PMID: 39438988
- PMCID: PMC11494811
- DOI: 10.1186/s13046-024-03180-y
Combining ERAP1 silencing and entinostat therapy to overcome resistance to cancer immunotherapy in neuroblastoma
Abstract
Background: Checkpoint immunotherapy unleashes tumor control by T cells, but it is undermined in non-immunogenic tumors, e.g. with low MHC class I expression and low neoantigen burden, such as neuroblastoma (NB). Endoplasmic reticulum aminopeptidase 1 (ERAP1) is an enzyme that trims peptides before loading on MHC class I molecules. Inhibition of ERAP1 results in the generation of new antigens able of inducing potent anti-tumor immune responses. Here, we identify a novel non-toxic combinatorial strategy based on genetic inhibition of ERAP1 and administration of the HDAC inhibitor (HDACi) entinostat that increase the immunogenicity of NB, making it responsive to PD-1 therapy.
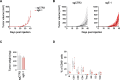
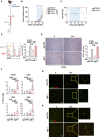
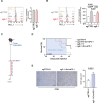
Methods: CRISPR/Cas9-mediated gene editing was used to knockout (KO) the ERAP1 gene in 9464D NB cells derived from spontaneous tumors of TH-MYCN transgenic mice. The expression of MHC class I and PD-L1 was evaluated by flow cytometry (FC). The immunopeptidome of these cells was studied by mass spectrometry. Cocultures of splenocytes derived from 9464D bearing mice and tumor cells allowed the assessment of the effect of ERAP1 inhibition on the secretion of inflammatory cytokines and activation and migration of immune cells towards ERAP1 KO cells by FC. Tumor cell killing was evaluated by Caspase 3/7 assay and flow cytometry analysis. The effect of ERAP1 inhibition on the immune content of tumors was analyzed by FC, immunohistochemistry and multiple immunofluorescence.
Results: We found that inhibition of ERAP1 makes 9464D cells more susceptible to immune cell-mediated killing by increasing both the recall and activation of CD4+ and CD8+ T cells and NK cells. Treatment with entinostat induces the expression of MHC class I and PD-L1 molecules in 9464D both in vitro and in vivo. This results in pronounced changes in the immunopeptidome induced by ERAP1 inhibition, but also restrains the growth of ERAP1 KO tumors in vivo by remodelling the tumor-infiltrating T-cell compartment. Interestingly, the absence of ERAP1 in combination with entinostat and PD-1 blockade overcomes resistance to PD-1 immunotherapy and increases host survival.
Conclusions: These findings demonstrate that ERAP1 inhibition combined with HDACi entinostat treatment and PD-1 blockade remodels the immune landscape of a non-immunogenic tumor such as NB, making it responsive to checkpoint immunotherapy.
Keywords: Antigen processing; Cancer immunotherapy; Combination therapy; ERAP1; Epigenetic; Immunopeptidome; Neuroblastoma.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Korman AJ, Garrett-Thomson SC, Lonberg N. The foundations of immune checkpoint blockade and the ipilimumab approval decennial. Nat Rev Drug Discov. 2022;21(7):509–28. - PubMed
-
- Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369(9579):2106–20. - PubMed
-
- London WB, Bagatell R, Weigel BJ, Fox E, Guo D, Van Ryn C, Naranjo A, Park JR. Historical time to disease progression and progression-free survival in patients with recurrent/refractory neuroblastoma treated in the modern era on Children’s Oncology Group early-phase trials. Cancer. 2017;123(24):4914–23. - PMC - PubMed
-
- Matthay KK, Maris JM, Schleiermacher G, Nakagawara A, Mackall CL, Diller L, Weiss WA. Neuroblastoma Nat Rev Dis Primers. 2016;2:16078. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

