A minimally invasive biomarker for sensitive and accurate diagnosis of Parkinson's disease
- PMID: 39439002
- PMCID: PMC11495072
- DOI: 10.1186/s40478-024-01873-1
A minimally invasive biomarker for sensitive and accurate diagnosis of Parkinson's disease
Abstract
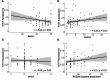
Seeding activities of disease-associated α-synuclein aggregates (αSynD), a hallmark of Parkinson's disease (PD), are detectable by seed amplification assay (αSyn-SAA) and being developed as a diagnostic biomarker for PD. Sensitive and accurate αSyn-SAA for blood or saliva would greatly facilitate PD diagnosis. This prospective diagnostic study conducted αSyn-SAA analyses on serum and saliva samples collected from patients clinically diagnosed with PD or healthy controls (HC). 124 subjects (82 PD, 42 HC) donated blood and had extensive clinical assessments, of whom 74 subjects (48 PD, 26 HC) also donated saliva at the same visits. An additional 57 subjects (35 PD, 22 HC) donated saliva and had more limited clinical assessments. The mean ages were 69.21, 66.55, 69.58, and 64.71 years for PD serum donors, HC serum donors, PD saliva donors, and HC saliva donors, respectively. αSynD seeding activities in either sample type alone or both sample types together were evaluated for PD diagnosis. Serum αSyn-SAA data from 124 subjects showed 80.49% sensitivity, 90.48% specificity, and 0.9006 accuracy (AUC of ROC); saliva αSyn-SAA data from 131 subjects attained 74.70% sensitivity, 97.92% specificity, and 0.8966 accuracy. Remarkably, the combined serum and saliva αSyn-SAA from 74 subjects with both sample types achieved better diagnostic performance: 95.83% sensitivity, 96.15% specificity, and 0.98 accuracy. In addition, serum αSynD seeding activities correlated inversely with Montreal Cognitive Assessment in males and positively with Hamilton Depression Rating Scale in females and in the < 70 age group, whereas saliva αSynD seeding activities correlated inversely with age at diagnosis in males and in the < 70 age group. Our data indicate that serum and saliva αSyn-SAA together can achieve high diagnostic accuracy for PD comparable to that of CSF αSyn-SAA, suggesting their potential utility for highly sensitive, accurate, and minimally invasive diagnosis of PD in routine clinical practice and clinical studies.
Keywords: Alpha-synuclein; Biomarker; Parkinson’s disease; RT-QuIC; Seeding activity.
© 2024. The Author(s).
Conflict of interest statement
Qingzhong Kong, Steven A. Gunzler, Zerui Wang and Shu G. Chen have received grants from the National Institutes of Health. Steven A. Gunzler received grants from the Michael J Fox Foundation (MJFF) and the Parkinson Foundation, and has participated in studies funded by Biogen, Amneal, Bial, and UCB. Zerui Wang received funding from MJFF. Shu G. Chen received funding from MJFF and CurePSP. No other disclosures were reported.
Figures




Update of
-
A minimally Invasive Biomarker for Sensitive and Accurate Diagnosis of Parkinson's Disease.medRxiv [Preprint]. 2024 Jun 30:2024.06.29.24309703. doi: 10.1101/2024.06.29.24309703. medRxiv. 2024. Update in: Acta Neuropathol Commun. 2024 Oct 22;12(1):167. doi: 10.1186/s40478-024-01873-1. PMID: 38978648 Free PMC article. Updated. Preprint.
References
-
- Hughes JE, Daniel SE, Ben-Shlomo Y, Lees AJ (2002) The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain 125(4):861–870 - PubMed
-
- Rizzo G, Copetti M, Arcuti S, Martino D, Fontana A, Logroscino G (2016) Accuracy of clinical diagnosis of Parkinson disease: a systematic review and meta-analysis. Neurology 86(6):566–576 - PubMed
-
- Virameteekul S, Revesz T, Jaunmuktane Z, Warner TT, De Pablo-Fernández E (2023) Clinical diagnostic accuracy of Parkinson’s disease: where do we stand? Mov Disord 38(4):558–566 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

