A Tympanic Piezo-Bioreactor Modulates Ion Channel-Associated Mechanosignaling to Stabilize Phenotype and Promote Tenogenesis in Human Tendon-Derived Cells
- PMID: 39439240
- PMCID: PMC11615817
- DOI: 10.1002/advs.202405711
A Tympanic Piezo-Bioreactor Modulates Ion Channel-Associated Mechanosignaling to Stabilize Phenotype and Promote Tenogenesis in Human Tendon-Derived Cells
Abstract
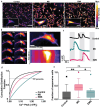
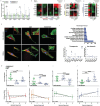
Preserving the function of human tendon-derived cells (hTDCs) during cell expansion is a significant challenge in regenerative medicine. In this study, a non-genetic approach is introduced to control the differentiation of hTDCs using a newly developed tympanic bioreactor. The system mimics the functionality of the human tympanic membrane, employing a piezoelectrically tuned acoustic diaphragm made of polyvinylidene fluoride-co-trifluoroethylene and boron nitride nanotubes. The diaphragm is vibrationally actuated to deliver targeted electromechanical stimulation to hTDCs. The results demonstrate that the system effectively maintains the tendon-specific phenotype of hTDCs, even under conditions that typically induce nonspecific differentiation, such as osteogenesis. This stabilization is achieved by modulating integrin-mediated mechanosignaling via ion channel-regulated calcium activity, potentially by TREK-1 and PIEZO1, yet targeted studies are required for confirmation. Finally, the system sustains the activation of key differentiation pathways (bone morphogenetic protein, BMP) while downregulating osteogenesis-associated (mitogen-ctivated protein kinase, MAPK and wingless integrated, WNT) pathways and upregulating Focal Adhesion Kinase (FAK) signaling. This approach offers a finely tunable, dose-dependent control over hTDC differentiation, presenting significant potential for non-genetic approaches in cell therapy, tendon tissue engineering, and the regeneration of other mechanosensitive tissues.
Keywords: BNNT; FAK; PVDF‐TrFE; electromechanical; focal adhesions; mechanotransduction; piezoelectricity; tendon.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Bi Y., Ehirchiou D., Kilts T. M., Inkson C. A., Embree M. C., Sonoyama W., Li Li, Leet A. I., Seo B.‐M., Zhang Li, Shi S., Young M. F., Nat. Med. 2007, 13, 1219. - PubMed
-
- Zhang J., Wang J. H., Methods Mol. Biol. 2018, 1842, 217. - PubMed
-
- Wei B., Lu J., Stem Cell Rev. Rep. 2021, 17, 1534. - PubMed
-
- Nature 2023, 616, 629. - PubMed
-
- Lomas A. J., Ryan C. N. M., Sorushanova A., Shologu N., Sideri A. I., Tsioli V., Fthenakis G. C., Tzora A., Skoufos I., Quinlan L. R., O'Laighin G., Mullen A. M., Kelly J. L., Kearns S., Biggs M., Pandit A., Zeugolis D. I., Adv. Drug Deliv. Rev. 2015, 84, 257. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
