Assessing clinical progression measures in Alzheimer's disease trials: A systematic review and meta-analysis
- PMID: 39439251
- PMCID: PMC11667530
- DOI: 10.1002/alz.14314
Assessing clinical progression measures in Alzheimer's disease trials: A systematic review and meta-analysis
Abstract
Introduction: Assessing treatments for Alzheimer's disease (AD) relies on reliable tools for measuring AD progression. In this analysis, we evaluate the sensitivity of clinical progression measures in AD within randomized controlled trials (RCTs) with confirmed positive amyloid (Aβ+) status prior to trial enrollment.
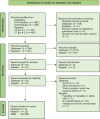
Methods: Excluding trials targeting non-cognitive symptoms, we conducted meta-analyses on progression measures from 25 selected RCTs using R version 4.2.0, along with the metafor and emmeans libraries.
Results: The Functional Activities Questionnaire (FAQ) demonstrated the greatest sensitivity over 12 weeks. Other cognitive measures demonstrated lower sensitivity. The integrated Alzheimer's Disease Rating Scale (iADRS) and Clinical Dementia Rating-Sum of Boxes (CDR-SB) seemed more effective than their individual cognitive components. Neuropsychiatric measures were the least sensitive in measuring progression.
Discussion: Functional measures generally outperformed other measure categories. Purely cognitive domain-based measures were suboptimal for tracking early AD progression. Ideally, future measures should incorporate both cognitive and functional components to enhance sensitivity.
Highlights: Concerns remain regarding the limitations of current outcome measures used in AD clinical trials, particularly their sensitivity in the early and preclinical stages of the disease, which hampers their reliability as indicators of AD progression. The Functional Activities Questionnaire (FAQ) demonstrated the most substantial weighted mean change over 12 weeks, followed by the Mini-Mental State Examination (MMSE). Functional measures outperformed other measure categories. Composite scores of integrated Alzheimer's Disease Rating Scale and Clinical Dementia Rating-Sum of Boxes are more sensitive to change than their individual cognitive components, possibly driven by the functional components of the score. Neuropsychiatric measures analyzed in this study appeared to be the least sensitive in measuring progression.
Keywords: Alzheimer's dementia; amyloid positive; meta‐analysis; outcome measures; progression measures; randomized controlled trials; systematic review.
© 2024 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declare no conflicts of interest. Author disclosures are available in the Supporting information.
Figures
References
-
- Mintun MA, Lo AC, Duggan Evans C, et al. Donanemab in early Alzheimer's disease. N Engl J Med. 2021;384(18):1691‐1704. - PubMed
-
- van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer's disease. N Engl J Med. 2023;388(1):9‐21. - PubMed
-
- Harris E. Alzheimer Drug Lecanemab Gains Traditional FDA Approval. JAMA. 2023;330(6):495. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical