Navigating directed evolution efficiently: optimizing selection conditions and selection output analysis
- PMID: 39439528
- PMCID: PMC11493728
- DOI: 10.3389/fmolb.2024.1439259
Navigating directed evolution efficiently: optimizing selection conditions and selection output analysis
Abstract
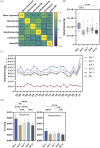
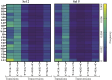
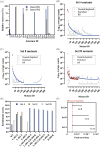
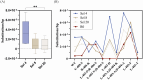
Directed evolution is a powerful tool that can bypass gaps in our understanding of the sequence-function relationship of proteins and still isolate variants with desired activities, properties, and substrate specificities. The rise of directed evolution platforms for polymerase engineering has accelerated the isolation of xenobiotic nucleic acid (XNA) synthetases and reverse transcriptases capable of processing a wide array of unnatural XNAs which have numerous therapeutic and biotechnological applications. Still, the current generation of XNA polymerases functions with significantly lower efficiency than the natural counterparts and retains a significant level of DNA polymerase activity which limits their in vivo applications. Although directed evolution approaches are continuously being developed and implemented to improve XNA polymerase engineering, the field lacks an in-depth analysis of the effect of selection parameters, library construction biases and sampling biases. Focusing on the directed evolution pipeline for DNA and XNA polymerase engineering, this work sets out a method for understanding the impact of selection conditions on selection success and efficiency. We also explore the influence of selection conditions on fidelity at the population and individual mutant level. Additionally, we explore the sequencing coverage requirements in directed evolution experiments, which differ from genome assembly and other -omics approaches. This analysis allowed us to identify the sequencing coverage threshold for the accurate and precise identification of significantly enriched mutants. Overall, this study introduces a robust methodology for optimizing selection protocols, which effectively streamlines selection processes by employing small libraries and cost-effective NGS sequencing. It provides valuable insights into critical considerations, thereby enhancing the overall effectiveness and efficiency of directed evolution strategies applicable to enzymes other than the ones considered here.
Keywords: design of experiments; directed evolution; fitness landscape; next-generation sequencing (NGS) data analysis; polymerase engineering.
Copyright © 2024 Handal-Marquez, Nguyen and Pinheiro.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Antony J. (2023). Design of experiments for engineers and scientists, third edition. Elsevier.
LinkOut - more resources
Full Text Sources

