Advancing non-alcoholic fatty liver disease prediction: a comprehensive machine learning approach integrating SHAP interpretability and multi-cohort validation
- PMID: 39439566
- PMCID: PMC11493712
- DOI: 10.3389/fendo.2024.1450317
Advancing non-alcoholic fatty liver disease prediction: a comprehensive machine learning approach integrating SHAP interpretability and multi-cohort validation
Abstract
Introduction: Non-alcoholic fatty liver disease (NAFLD) represents a major global health challenge, often undiagnosed because of suboptimal screening tools. Advances in machine learning (ML) offer potential improvements in predictive diagnostics, leveraging complex clinical datasets.
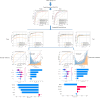
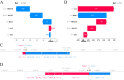
Methods: We utilized a comprehensive dataset from the Dryad database for model development and training and performed external validation using data from the National Health and Nutrition Examination Survey (NHANES) 2017-2020 cycles. Seven distinct ML models were developed and rigorously evaluated. Additionally, we employed the SHapley Additive exPlanations (SHAP) method to enhance the interpretability of the models, allowing for a detailed understanding of how each variable contributes to predictive outcomes.
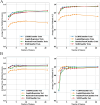
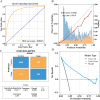
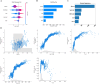
Results: A total of 14,913 participants were eligible for this study. Among the seven constructed models, the light gradient boosting machine achieved the highest performance, with an area under the receiver operating characteristic curve of 0.90 in the internal validation set and 0.81 in the external NHANES validation cohort. In detailed performance metrics, it maintained an accuracy of 87%, a sensitivity of 92.9%, and an F1 score of 0.92. Key predictive variables identified included alanine aminotransferase, gammaglutamyl transpeptidase, triglyceride glucose-waist circumference, metabolic score for insulin resistance, and HbA1c, which are strongly associated with metabolic dysfunctions integral to NAFLD progression.
Conclusions: The integration of ML with SHAP interpretability provides a robust predictive tool for NAFLD, enhancing the early identification and potential management of the disease. The model's high accuracy and generalizability across diverse populations highlight its clinical utility, though future enhancements should include longitudinal data and lifestyle factors to refine risk assessments further.
Keywords: SHAP interpretability; light gradient boosting machine; machine learning; non-alcoholic fatty liver disease; predictive model.
Copyright © 2024 Yang, Lu and Ran.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

