Role of SARS-CoV-2-specific memory B cells promoting immune protection after booster vaccination in solid organ transplantation
- PMID: 39439787
- PMCID: PMC11493670
- DOI: 10.3389/fimmu.2024.1463769
Role of SARS-CoV-2-specific memory B cells promoting immune protection after booster vaccination in solid organ transplantation
Abstract
Introduction: Solid organ transplant (SOT) recipients display weak seroconversion and neutralizing antibody (NAb) responses after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination and remain at risk of severe coronavirus disease 2019 (COVID-19). While B-cell memory is the hallmark of serological immunity, its role in driving successful vaccine responses and providing immune protection in SOT patients remains unclear.
Methods: We investigated the function and interplay of SARS-CoV-2-specific memory B cells (mBc), different cytokineproducing T cells, and cross-reactive NAb in driving seroconversion and protection against COVID-19 in two cohorts. First, we studied a large cohort of 148 SOT recipients and 32 immunocompetent individuals who underwent several vaccinations. Subsequently, we assessed 25 SOT patients participating in a randomized controlled trial to compare two different immunosuppressive strategies for allowing successful seroconversion and memory-cell responses after booster vaccination.
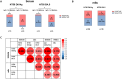
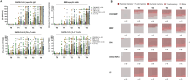
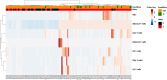
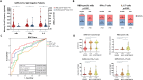
Results: We corroborate previous findings that B- and T-cell memory responses are weaker and more delayed in SOT patients than in immunocompetent (IC) individuals; however, within the SOT cohort, we found that these responses are relatively stronger and more robust in patients not receiving mycophenolate mofetil (MMF)-based therapies. Anti- spike IgG titers strongly correlated with RBD-specific IgG-producing mBc, with both displaying broad viral cross reactivity. Prebooster SARS-CoV-2-specific mBc and IL-2- producing T cells accurately predicted Nab seroconversion (AUC, 0.828) and protection against severe COVID-19. While switching unresponsive SOT patients from calcineurin inhibitors (CNI)/MMF to a low-exposure CNI/mTOR-i regimen favored wider SARS-CoV-2-specific immune responses after a fourth booster vaccination, preformed RBD-specific mBc predicted NAb seroconversion.
Discussion: Our study adds new insights into the pathobiology of immune memory and highlights the pivotal role of SARS-CoV-2-specific mBc in promoting immune protection inSOT patients.
Keywords: SARS-CoV-2; adaptive immunity; booster vaccination; neutralizing antibodies; solid organ transplantation.
Copyright © 2024 Donadeu, Gomez-Olles, Casanova, Torija, Lopez-Meseguer, Boada-Pérez, Kervella, Crespo, Carrera-Muñoz, Campos-Varela, Castells, Cortese, Esperalba, Fernández-Naval, Quintero, Muñoz, Agüero, Gonzalez-Costello, Lladó, Favà, Cañas, del Mar de la Hoz-Caballero, Meneghini, Torres, Juvé, Hafkamp, Vila, Robles, Buzón, Toapanta, Zúñiga, Monforte, Saez-Giménez, Len, Arcos, Miret, Ariceta, Pardo, Martínez, Moreso and Bestard.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



References
-
- European Centre for Disease Prevention and Control. Interim public health considerations for the provision of additional COVID-19 vaccine doses (2021). ECDC: Stockholm; (Accessed 1 September 2021).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

