Accessing the synthesis of natural products and their analogues enabled by the Barbier reaction: a review
- PMID: 39439835
- PMCID: PMC11495476
- DOI: 10.1039/d4ra05646a
Accessing the synthesis of natural products and their analogues enabled by the Barbier reaction: a review
Abstract
The Barbier reaction is significantly referred to as one of the efficient carbon-carbon bond forming reactions which involves the treatment of haloalkanes and carbonyl compounds by utilizing the catalytic role of a diverse range of metals and metalloids. The Barbier reaction is tolerant to a variety of functional groups, allowing a broad substrate scope with the employment of lanthanides, transition metals, amphoteric elements or alkaline earth metals. This reaction is also water-resistant, thereby overcoming the challenges posed by moisture sensitive organometallic species involving C-C bond formation reactions. The Barbier reaction has significantly found its applicability towards the synthesis of intricate and naturally occurring organic compounds. Our review provides an outlook on the synthetic applications of the Barbier reaction and its variants to accomplish the preparation of several natural products, reported since 2020.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


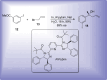
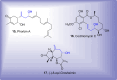
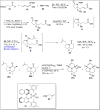

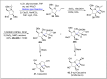
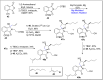
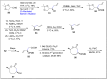
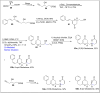
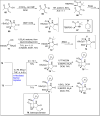
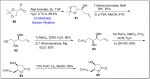

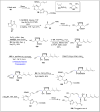

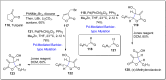



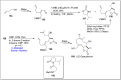
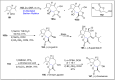

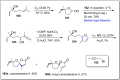

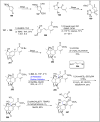
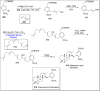
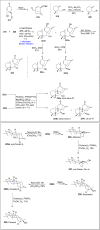
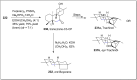
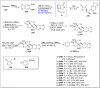
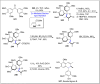
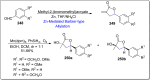


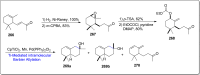

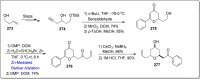
References
-
- Mayr H. Kempf B. Ofial A. R. π-Nucleophilicity in carbon–carbon bond-forming reactions. Acc. Chem. Res. 2003;36:66–77. - PubMed
-
- Ravelli D. Protti S. Fagnoni M. Carbon–carbon bond forming reactions via photogenerated intermediates. Chem. Rev. 2016;116:9850–9913. - PubMed
-
- Brahmachari G. Design for carbon–carbon bond forming reactions under ambient conditions. RSC Adv. 2016;6:64676–64725.
-
- Nair V. Vellalath S. Babu B. P. Recent advances in carbon–carbon bond-forming reactions involving homoenolates generated by NHC catalysis. Chem. Soc. Rev. 2008;37:2691–2698. - PubMed
-
- Wu X. F. Neumann H. Zinc-Catalyzed Organic Synthesis: C-C, C-N, C-O Bond Formation Reactions. Adv. Synth. Catal. 2012;354:3141–3160.
Publication types
LinkOut - more resources
Full Text Sources

