The significance of chirality in contemporary drug discovery-a mini review
- PMID: 39439836
- PMCID: PMC11495282
- DOI: 10.1039/d4ra05694a
The significance of chirality in contemporary drug discovery-a mini review
Abstract
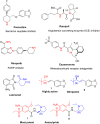
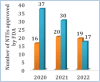
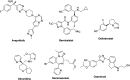
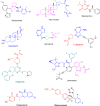
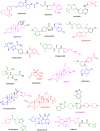
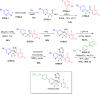
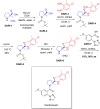
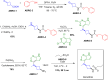
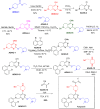
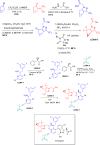
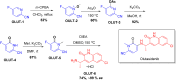
More than half of drugs are chiral compounds with their chirality determining their molecular interactions, ecofriendly environmental safety and efficacy. Overall nearly 90% of chiral compounds are marketed as racemates consisting of an equimolar mixture of two enantiomers. Despite having identical chemical structure and bonding, racemates function differently when exposed to chiral environments and demonstrate notable variances in biological properties such as pharmacology, toxicology, metabolism and pharmacokinetics, etc. Advancements in asymmetric synthesis in recent years have led to considerable interest in the development of single enantiomers of chiral drug molecules for medicinal chemistry settings. In this review, we want to compile examples of chiral medicines approved by the FDA in the years 2022 and 2023 with an emphasis on their synthesis along with information on chiral induction as well as enantiomeric excess.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that there are no conflicts of interests.
Figures



Similar articles
-
Chiral drugs: an overview.Int J Biomed Sci. 2006 Jun;2(2):85-100. Int J Biomed Sci. 2006. PMID: 23674971 Free PMC article.
-
From barbiturates to ganaxolone: The importance of chirality in drug development and in understanding the actions of old and new antiseizure medications.Pharmacol Ther. 2025 Apr;268:108808. doi: 10.1016/j.pharmthera.2025.108808. Epub 2025 Feb 5. Pharmacol Ther. 2025. PMID: 39920975 Review.
-
[Preparations and biological properties of chiral compounds].Arh Hig Rada Toksikol. 2005 Dec;56(4):351-61. Arh Hig Rada Toksikol. 2005. PMID: 16370519 Review. Croatian.
-
Asymmetric Synthesis of US-FDA Approved Drugs over Five Years (2016-2020): A Recapitulation of Chirality.Pharmaceuticals (Basel). 2023 Feb 22;16(3):339. doi: 10.3390/ph16030339. Pharmaceuticals (Basel). 2023. PMID: 36986439 Free PMC article. Review.
-
Stereochemistry and drug efficacy and development: relevance of chirality to antidepressant and antipsychotic drugs.Ann Med. 2002;34(7-8):537-43. doi: 10.1080/078538902321117742. Ann Med. 2002. PMID: 12553493 Review.
Cited by
-
Mitsunobu reaction: assembling C-N bonds in chiral traditional Chinese medicine.RSC Adv. 2025 Feb 17;15(7):5167-5189. doi: 10.1039/d4ra08573f. eCollection 2025 Feb 13. RSC Adv. 2025. PMID: 39963451 Free PMC article. Review.
-
Exploring the Impact of Chirality of Synthetic Cannabinoids and Cathinones: A Systematic Review on Enantioresolution Methods and Enantioselectivity Studies.Int J Mol Sci. 2025 Jul 4;26(13):6471. doi: 10.3390/ijms26136471. Int J Mol Sci. 2025. PMID: 40650252 Free PMC article.
-
All-Heteroatom-Substituted Carbon Spiro Stereocenters: Synthesis, Resolution, Enantiomeric Stability, and Absolute Configuration.J Am Chem Soc. 2025 Jun 18;147(24):21121-21130. doi: 10.1021/jacs.5c06394. Epub 2025 Jun 4. J Am Chem Soc. 2025. PMID: 40464057 Free PMC article.
-
Sonosynthesis of new functionalized optically active triazines via double Mannich reaction: antibacterial potential and in silico docking study.RSC Adv. 2025 Jun 4;15(22):17516-17534. doi: 10.1039/d5ra01283j. eCollection 2025 May 21. RSC Adv. 2025. PMID: 40469558 Free PMC article.
-
CALB Immobilized on Octyl-Agarose-An Efficient Pharmaceutical Biocatalyst for Transesterification in Organic Medium.Int J Mol Sci. 2025 Jul 20;26(14):6961. doi: 10.3390/ijms26146961. Int J Mol Sci. 2025. PMID: 40725207 Free PMC article.
References
-
- Food and Drug Administration, Development of New Stereoisomeric Drugs, Rockville, MD, 1992
-
- Calcaterra A. D'Acquarica I. J. Pharm. Biomed. Anal. 2018;147:323–340. - PubMed
-
- Agranat I. Wainschtein S. R. Zusman E. Z. Nat. Rev. Drug Discovery. 2012;11:972–973. - PubMed
-
- Ishikawa M. Hashimoto Y. J. Med. Chem. 2011;54:1539–1554. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

