Validation of Modified Objective Prognostic Score in Patients with Advanced Cancer in Taiwan
- PMID: 39440111
- PMCID: PMC11491570
- DOI: 10.1089/pmr.2024.0036
Validation of Modified Objective Prognostic Score in Patients with Advanced Cancer in Taiwan
Abstract
Background: Modified versions of the Objective Prognostic Score (mOPS) needs to be validated to reflect practical palliative care circumstances in Taiwan.
Objectives: We compared the abilities of an mOPS score of 1.5 or higher versus a Karnofsky Performance Status (KPS) score of 30 or lower to predict 2-week mortality in patients with advanced cancer in Taiwan.
Design: Observational study.
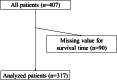
Setting/subjects: We performed a secondary analysis of an international multicenter cohort study of patients in East Asia. Participants were inpatients with advanced cancer in palliative care units (PCUs) in Taiwan.
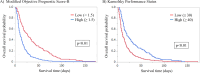
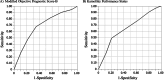
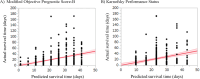
Measurements: We compared the mOPS-B model, which does not require laboratory tests, with the KPS in a 2-week survival timeframe. We compared the accuracy of the prognostic models using sensitivity, specificity, and area under the receiver operating characteristic curve (AUROC). Calibration plots and net reclassification indices (NRI) for 2-week survival were compared between the two models. Differences in survival between the higher- and lower-scoring groups of each model were identified using the log-rank test.
Results: We included 317 patients, with a median survival of 14.0 days. The mOPS-B had a high sensitivity (0.82) and high AUROC value (0.69). By contrast, the KPS demonstrated good sensitivity (0.77) and an acceptable AUROC value (0.65) for predicting 2-week survival. The calibration plot did not demonstrate satisfactory agreement between the actual and predicted survival times in either the mOPS-B or the KPS groups. Our NRI was positive (absolute value: 22%), indicating that mOPS-B predicted 2-week survival better than KPS.
Conclusions: The mOPS-B may serve better than the KPS as a screening tool for admission to PCUs in Taiwan because it was more accurate at predicting 2-week survival.
Keywords: advanced cancer; palliative care; prognostication; validity.
© The Author(s) 2024. Published by Mary Ann Liebert, Inc.
Figures
References
-
- Stone P, Buckle P, Dolan R, et al. ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org . Prognostic evaluation in patients with advanced cancer in the last months of life: ESMO clinical practice guideline. ESMO Open 2023;8(2):101195; doi:10.1016/j.esmoop.2023.101195 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
