Axonal damage and inflammation response are biological correlates of decline in small-world values: a cohort study in autosomal dominant Alzheimer's disease
- PMID: 39440304
- PMCID: PMC11495221
- DOI: 10.1093/braincomms/fcae357
Axonal damage and inflammation response are biological correlates of decline in small-world values: a cohort study in autosomal dominant Alzheimer's disease
Abstract
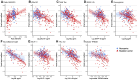
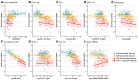
The grey matter of the brain develops and declines in coordinated patterns during the lifespan. Such covariation patterns of grey matter structure can be quantified as grey matter networks, which can be measured with magnetic resonance imaging. In Alzheimer's disease, the global organization of grey matter networks becomes more random, which is captured by a decline in the small-world coefficient. Such decline in the small-world value has been robustly associated with cognitive decline across clinical stages of Alzheimer's disease. The biological mechanisms causing this decline in small-world values remain unknown. Cerebrospinal fluid (CSF) protein biomarkers are available for studying diverse pathological mechanisms in humans and can provide insight into decline. We investigated the relationships between 10 CSF proteins and small-world coefficient in mutation carriers (N = 219) and non-carriers (N = 136) of the Dominantly Inherited Alzheimer Network Observational study. Abnormalities in Amyloid beta, Tau, synaptic (Synaptosome associated protein-25, Neurogranin) and neuronal calcium-sensor protein (Visinin-like protein-1) preceded loss of small-world coefficient by several years, while increased levels in CSF markers for inflammation (Chitinase-3-like protein 1) and axonal injury (Neurofilament light) co-occurred with decreasing small-world values. This suggests that axonal loss and inflammation play a role in structural grey matter network changes.
Keywords: autosomal dominant Alzheimer disease; axonal damage; inflammation; neuronal injury; structural covariance network.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
C.C.: receives research support from: Biogen, EISAI, Alector and Parabon. The funders of the study had no role in the collection, analysis, interpretation of data; or in the writing of the report; or in the decision to submit the paper for publication. C.C. is a member of the advisory board of ADx Healthcare and Vivid Genomics Marty Farlow: Grant/Research Support from AbbVie, ADCS Posiphen, AstraZeneca, Biogen, Eisai, Eli Lilly, Genentech, Novartis, Suven Life Sciences, Ltd., vTv Therapeutics; Consultant/Advisory Boards/DSMB Boards for Allergan, Avanir, AZTherapies, Biogen MA Inc., Cerecin (formerly Accera), Chemigen, Cognition Therapeutics, Cortexyme, Danone, Eisai Inc., Eli Lilly, Longeveron, Green Valley, Medavante, Otsuka Pharmaceutical, Proclara, Neurotrope Bioscience, Samumed, Takeda, vTv Therapeutics, Zhejian Hisun Pharmaceuticals; patent Elan Johannes Levin reports speaker’s fees from Bayer Vital, speaker’s fees from Willi Gross Foundation, consulting fees from Axon Neuroscience, consulting fees from Ionis Pharmaceuticals, non-financial support from Abbvie, MODAG compensation for part time CMO, Thieme medical publishers and W. Kohlhammer GmbH medical publishers author fees, outside the submitted work. E.M.: NIA (research Funding); Eli Lilly-DSMB; ESAI—CMS; Alzamend—scientific advisory board. P.S. is partner at LSP Invester fund and has acquired grant support (for the institution) from GE Healthcare, Danone Research, Piramal and Merck. In the past 2 years, he has received consultancy/speaker fees (paid to the institution) from Lilly, GE Healthcare, Novartis, Sanofi, Nutricia, Probiodrug, Biogen, Roche, Avraham and EIP Pharma. Outside of this manuscript, R.J.B. reports grant/research/clinical trial support: NIH, Alzheimer's Association, BrightFocus Foundation, Rainwater Foundation Tau Consortium, Association for Frontotemporal Degeneration, Cure Alzheimer's Fund, the Tau SILK Consortium (AbbVie, Biogen and Eli Lilly), Janssen, and an anonymous foundation. R.J.B. reports consulting fees/honoraria from Janssen, Pfizer, Roche, Eisai, and Merck. R.J.B. reports equity ownership interest/advisory board income from C2N Diagnostics. All other authors report no disclosures.
Figures

References
-
- Tijms BM, Series P, Willshaw DJ, Lawrie SM. Similarity-based extraction of individual networks from gray matter MRI scans. Cereb Cortex. 2012;22(7):1530–1541. - PubMed
-
- Tijms BM, Wink AM, de Haan W, et al. . Alzheimer's disease: Connecting findings from graph theoretical studies of brain networks. Neurobiol Aging. 2013;34(8):2023–2036. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
