DNA-PK Inhibition Shows Differential Radiosensitization in Orthotopic GBM PDX Models Based on DDR Pathway Deficits
- PMID: 39440433
- PMCID: PMC12014860
- DOI: 10.1158/1535-7163.MCT-24-0003
DNA-PK Inhibition Shows Differential Radiosensitization in Orthotopic GBM PDX Models Based on DDR Pathway Deficits
Abstract
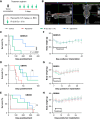
Glioblastoma (GBM) remains one of the most therapy-resistant malignancies with frequent local failures despite aggressive surgery, chemotherapy, and ionizing radiation (IR). Small molecule inhibitors of DNA-dependent protein kinase (DNA-PKi) are potent radiosensitizers currently in clinical trials. Determining which patients may benefit from radiosensitization with DNA-PKi is critical to avoid unnecessary increased risk of normal tissue toxicity. In this study, we used GBM patient-derived xenografts (PDX) in orthotopic murine models to study the relationship between molecular features, pharmacokinetics, and the radiosensitizing potential of the DNA-PKi peposertib. We show that peposertib radiosensitizes established and PDX GBM lines in vitro at 300 nmol/L and above, with a significant increase in radiosensitization by maintaining post-IR exposure for >12 hours. Radiosensitization by peposertib is mediated by catalytic inhibition of DNA-PK, and knockdown of DNA-PK by short hairpin RNA (shRNA) largely abolished the radiosensitizing effect. Peposertib decreased auto-phosphorylation of DNA-PKcs after IR in a dose-dependent manner with a delay in resolution of γH2AX foci at 24 hours. The addition of peposertib to IR significantly increased survival in GBM120 orthotopic xenografts, but not in GBM10. There was no difference in plasma or average tumor concentrations of peposertib in the two cohorts. Although the mechanism underpinning this discordant effect in vitro versus in vivo is not clear, there was an association for greater sensitization in TP53 mutant lines. Transfection of a dominant-negative TP53 mutant in baseline TP53 wild-type GBM lines significantly delayed growth and decreased nonhomologous end joining efficiency (but not homologous recombination), after peposertib exposure. See related commentary by Buchsbaum, p. 840.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
J.E. Eckel-Passow reports grants from NIH during the conduct of the study. N.Y.R. Agar reports grants from NIH during the conduct of the study; nonfinancial support from Bruker, nonfinancial support from Thermo, other support from EMD Serono, other support from iTeos Therapeutics, and other support from National Brain Tumor Society outside the submitted work; in addition, N.Y.R. Agar has a patent for U.S. provisional application No. 63/273,863 pending. Z.D. Nagel reports grants from the American Cancer Society during the conduct of the study; in addition, Z.D. Nagel has a patent for US9938587B2 issued. J.N. Sarkaria reports grants from the National Brain Tumor Society during the conduct of the study, as well as grants from Glaxo-Smith-Kline, Bayer, Wayshine, Black Diamond, Karyopharm, Boston Scientific, Wugen, Rain Therapeutics, Sumitomo Dainippon Pharma Oncology, AbbVie, SKBP, Boehringer Ingelheim, AstraZeneca, ABL Bio, ModifiBio, Inhibrx, Otomagnetics, Reglagene, and Breakpoint Therapeutics outside the submitted work. No disclosures were reported by the other authors.
Figures



References
-
- Zenke FT, Zimmermann A, Sirrenberg C, Dahmen H, Kirkin V, Pehl U, et al. Pharmacologic inhibitor of DNA-PK, M3814, potentiates radiotherapy and regresses human tumors in mouse models. Mol Cancer Ther 2020;19:1091–101. - PubMed
-
- Samuels M, Falkenius J, Bar-Ad V, Dunst J, van Triest B, Yachnin J, et al. A phase 1 study of the DNA-PK inhibitor peposertib in combination with radiation therapy with or without cisplatin in patients with advanced head and neck tumors. Int J Radiat Oncol Biol Phys 2024;118:743–56. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

