Salt-Responsive Gut Microbiota Induces Sex-Specific Blood Pressure Changes
- PMID: 39440438
- PMCID: PMC11905770
- DOI: 10.1161/CIRCRESAHA.124.325056
Salt-Responsive Gut Microbiota Induces Sex-Specific Blood Pressure Changes
Abstract
Background: Tryptophan metabolism is important in blood pressure regulation. The tryptophan-indole pathway is exclusively mediated by the gut microbiota. ACE2 (angiotensin-converting enzyme 2) participates in tryptophan absorption, and a lack of ACE2 leads to changes in the gut microbiota. The gut microbiota has been recognized as a regulator of blood pressure. Furthermore, there is ample evidence for sex differences in the gut microbiota. However, it is unclear whether such sex differences impact blood pressure differentially through the tryptophan-indole pathway.
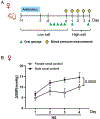
Methods: To study the sex-specific mechanisms of gut microbiota-mediated tryptophan-indole pathway in hypertension, we generated a novel rat model with Clustered Regularly Interspaced Short Palindromic Repeats/Cas9 (Clustered Regularly Interspaced Short Palindromic Repeats-associated protein 9)-targeted deletion of Ace2 in the Dahl salt-sensitive rat. Cecal microbiota transfers from donors of both sexes to female S recipients were performed. Also, Dahl salt-sensitive rats of both sexes were orally gavaged with indole to investigate blood pressure response.
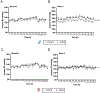
Results: The female gut microbiota and its tryptophan-indole pathway exhibited greater buffering capacity when exposed to tryptophan, due to Ace2 deficiency, and salt. In contrast, the male gut microbiota and its tryptophan-indole pathway were more vulnerable. Female rats with male cecal microbiota responded to salt with a higher blood pressure increase compared with those with female cecal microbiota. Indole, a tryptophan-derived metabolite produced by gut bacteria, increased blood pressure in male but not in female rats. Moreover, salt altered host-mediated tryptophan metabolism, characterized by reduced serum serotonin of both sexes and higher levels of kynurenine derivatives in the females.
Conclusions: We uncovered a novel sex-specific mechanism in the gut microbiota-mediated tryptophan-indole pathway in blood pressure regulation. Salt tipped the tryptophan metabolism between the host and gut microbiota in a sex-dependent manner. Our study provides evidence for a novel concept that gut microbiota and its metabolism play sex-specific roles in the development of salt-sensitive hypertension.
Keywords: blood pressure; gastrointestinal microbiome; hypertension; salts; sex characteristics.
Conflict of interest statement
None.
Figures







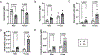
Comment in
-
Salt's Sex-Specific Impact of Gut Microbiota in Hypertension.Circ Res. 2024 Dec 6;135(12):1138-1140. doi: 10.1161/CIRCRESAHA.124.325719. Epub 2024 Dec 5. Circ Res. 2024. PMID: 39636968 Free PMC article. No abstract available.
References
-
- Lark LA, Witt PA, Becker KB, Studzinski WM, Weyhenmeyer JA. Effect of dietary tryptophan on the development of hypertension in the Dahl salt-sensitive rat. Clinical and Experimental Hypertension Part A: Theory and Practice. 1990;12:1–13. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

