Synthetic Control of Water-Stable Hybrid Perovskitoid Semiconductors
- PMID: 39440594
- PMCID: PMC12204132
- DOI: 10.1002/adma.202406274
Synthetic Control of Water-Stable Hybrid Perovskitoid Semiconductors
Abstract
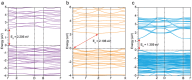
Hybrid metal-halide perovskites and their derived materials have emerged as the next-generation semiconductors with a wide range of applications, including photovoltaics, light-emitting devices, and other optoelectronics. Over the past decade, numerous single-crystalline perovskite derivatives have been synthesized and developed. However, the synthetic methods for these derivatives mainly rely on acidic crystallization conditions. This approach leads to crystals comprising metal halide building blocks, which show problematic stability when directly exposed to water. In this study, a methodology is developed for synthesizing hybrid metal-halide compounds using lead iodide and the zwitterionic bifunctional molecule cysteamine (CYS), to form various perovskitoid structures under a broad pH range. Interestingly, the different pH conditions alter the coordination environment of lead halides, leading to lead-sulfide and lead-nitride covalent bond formation. This modification significantly enhances their stability when in direct contact with water, lasting for months. Photoluminescence measurements and first principal density functional theory (DFT) calculations reveal that the perovskitoids synthesized under basic and acidic pH conditions exhibit a direct bandgap nature, while those synthesized under neutral conditions display an indirect bandgap. This approach opens new avenues for manipulating synthetic methods to develop water-stable hybrid semiconductors suitable for a wide range of applications, such as solid-state light emitters.
Keywords: halide perovskites; light emission; perovskitoids; single crystals; solid state semiconductor.
© 2024 The Author(s). Advanced Materials published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Steele J. A., Solano E., Jin H., Prakasam V., Braeckevelt T., Yuan H., Lin Z., de Kloe R., Wang Q., Rogge S. M. J., Van Speybroeck V., Chernyshov D., Hofkens J., Roeffaers M. B. J., Adv. Mater. 2021, 33, 2007224. - PubMed
-
- Nie W., Tsai H., Asadpour R., Blancon J.‐C., Neukirch A. J., Gupta G., Crochet J. J., Chhowalla M., Tretiak S., Alam M. A., Science 2015, 347, 522. - PubMed
-
- Poglitsch A., Weber D., J. Chem. Phys. 1987, 87, 6373.
Grants and funding
- FA9550-16-1-0358/Air Force Office of Scientific Research DURIP
- L. C. Hassinger Fellowship
- CHE-1726077/National Science Foundation
- DE-AC02-05CH11231/Laboratory Directed Research and Development Program of Lawrence Berkeley National Laboratory
- DE-AC02-05CH11231/Office of Science of the U.S. Department of Energy
LinkOut - more resources
Full Text Sources
Miscellaneous

